Fluorene derivatives having multi-photon absorption characteristic, synthesis method and applications thereof
A multi-photon absorption and synthesis method technology, which is applied in the field of fluorene derivatives with three-photon absorption characteristics and its preparation, can solve the problems that three-photon absorption materials are rarely reported, and achieve reasonable design, high stability, multi-photon absorption The effect of large cross-section
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
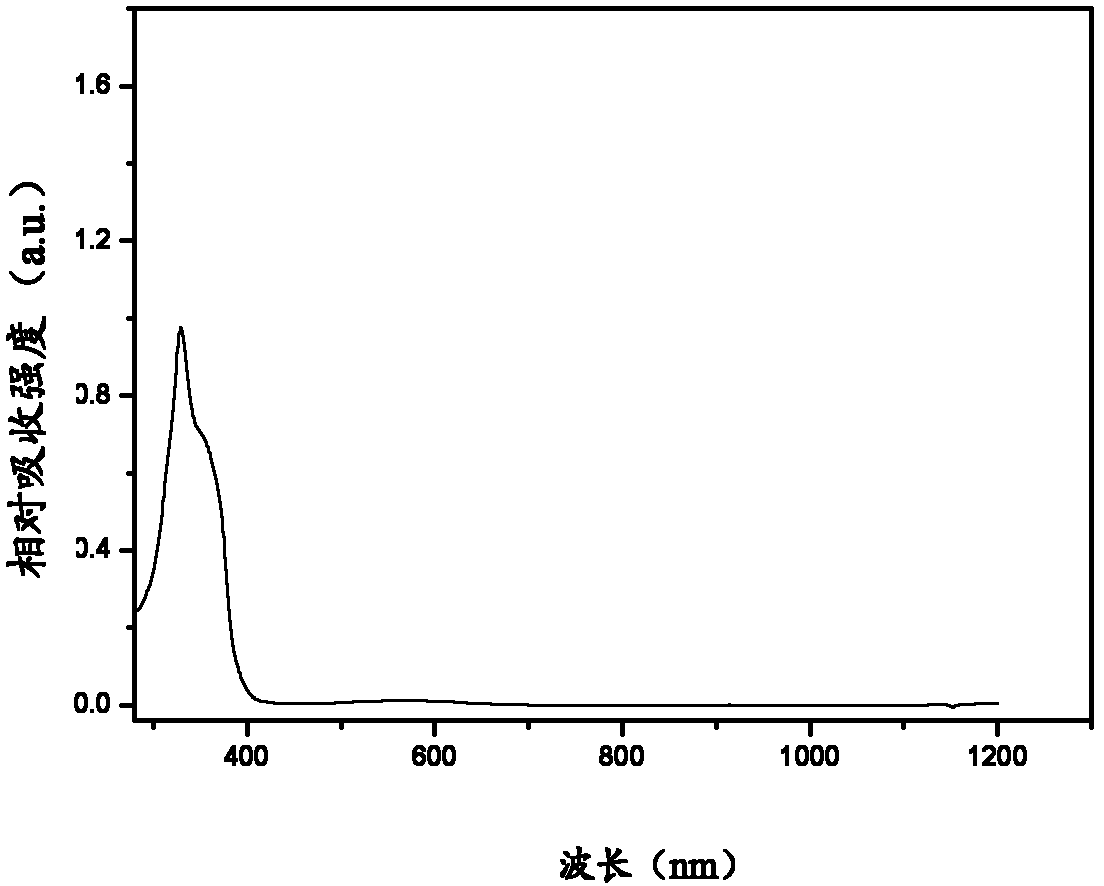
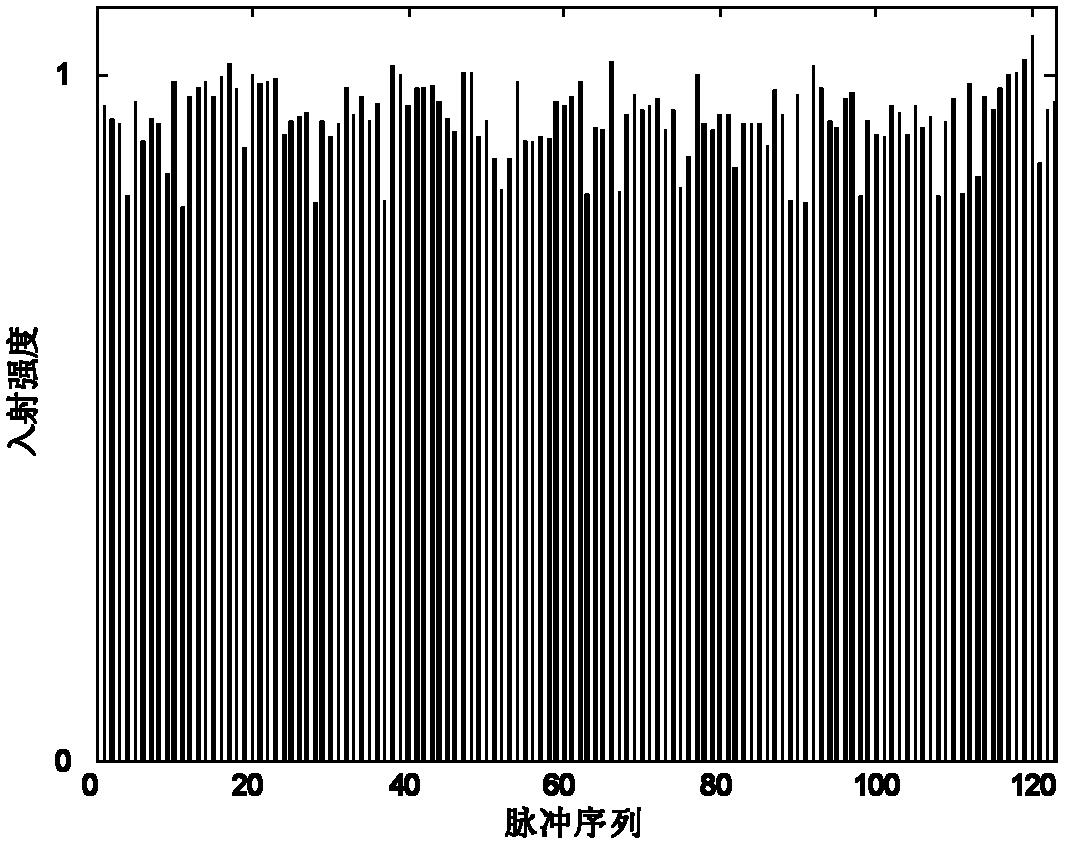
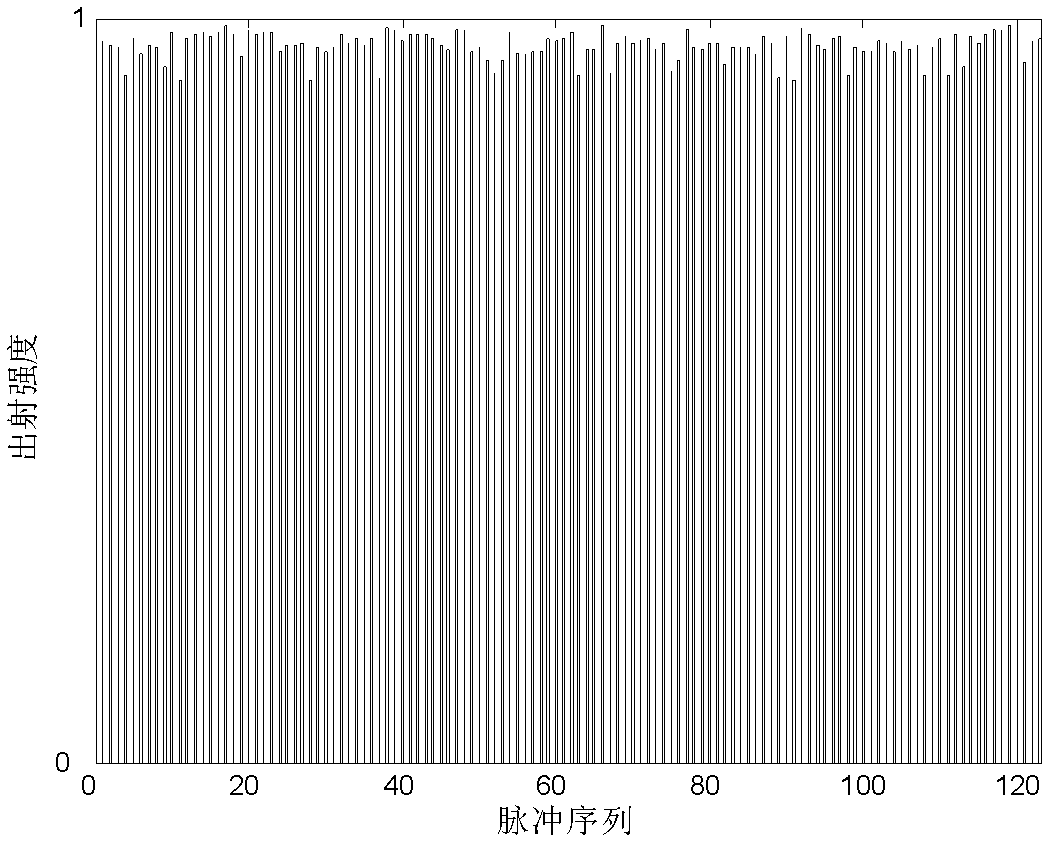
Image
Examples
Embodiment 1
[0028] The synthesis steps of 2,7-bis(4-methoxyphenylacetylene)-9-thione fluorene:
[0029] (1) Mix 2.5mmol potassium hydroxide and 200mmol fluorene into a beaker, gradually add 55°C dimethyl sulfoxide and stir until it is completely dissolved, keep the solution temperature at 55°C, pass in air, stir for 4 hours, and then react The solution was poured into water (the amount of water was 3 times the volume of the solution), and a yellow precipitate was precipitated, which was filtered, washed, dried, and recrystallized in ethanol to obtain 9-fluorenone with a yield of 83%;
[0030] (2) Mix 50mmol of fluorenone and 0.15mmol of iodine, add it to 80mL of water, slowly add 120mmol of bromine dropwise while stirring, heat and reflux for 7 hours, after the reaction is completed, wash with saturated aqueous sodium bisulfite until the red color fades, and then Washed 4 times with distilled water and recrystallized in ethanol to obtain 2,7-dibromofluorenone with a yield of 55%;
[0031...
Embodiment 2
[0034] The synthetic method of 2,7-bis(4-methoxyphenylacetylene)-9-thione fluorene is the same as that in Example 1, except that the ratio of raw materials and reaction conditions in each step are as follows:
[0035] (1) 2mmol potassium hydroxide and 200mmol fluorene were mixed and put into a beaker, the reaction temperature was 40°C, and after stirring for 5 hours, the yield was 75%;
[0036] (2) Add 50mmol fluorenone and 0.13mmol iodine to 90mL water, add 150mmol bromine dropwise, heat and reflux for 15 hours, yield 50%;
[0037] (3) Add 2.5mmol Pd(OAc) to a reaction flask 2 , 5mmolPPH 3、 150mL mixed solution of water and acetonitrile (volume ratio 1:8); add 20mmol 2,7-dibromofluorenone, 50mmol 4-methoxyphenylacetylene, 50mmol Bu 4 NHSO 4 , 300mmol triethylamine, 150mL mixed solution of water and acetonitrile (volume ratio is 1:8). Reaction at 25°C for 5 hours, yield 50%;
[0038] (4) 5 mmol of 2,7-bis(methoxyphenylacetylene)-9-fluorenone and 10 mmol of Lawson's reagen...
Embodiment 3
[0040] The synthetic method of 2,7-bis(4-n-pentoxyphenylacetylene)-9-thione fluorene is the same as that in Example 1, except that the raw materials, proportioning and reaction conditions in each step are different:
[0041] (1) Mix 3mmol potassium hydroxide and 200mmol fluorene into a beaker, the reaction temperature is 45°C, and after stirring for 5 hours, the yield is 80%;
[0042] (2) Add 50mmol of fluorenone and 0.17mmol of iodine to 90mL of water, add 150mmol of bromine dropwise, heat and reflux for 10 hours, yield 51%;
[0043] (3) 5mmol Pd(OAc) 2 , 5mmol PPH 3 Add a reaction flask, then add 150mL of water and acetonitrile mixed solution (volume ratio is 1:10); add 20mmol 2,7-dibromofluorenone, 50mmol 4-n-pentoxyphenylacetylene, 70 mmol Bu 4 NHSO 4 , 400 mmol triethylamine, and then add 150 mL of a mixed solution of water and acetonitrile (volume ratio is 1:10). Reaction at 30°C for 10 hours, yield 85%;
[0044] (4) 5 mmol of 2,7-bis(4-n-pentyloxyphenylacetylene)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com