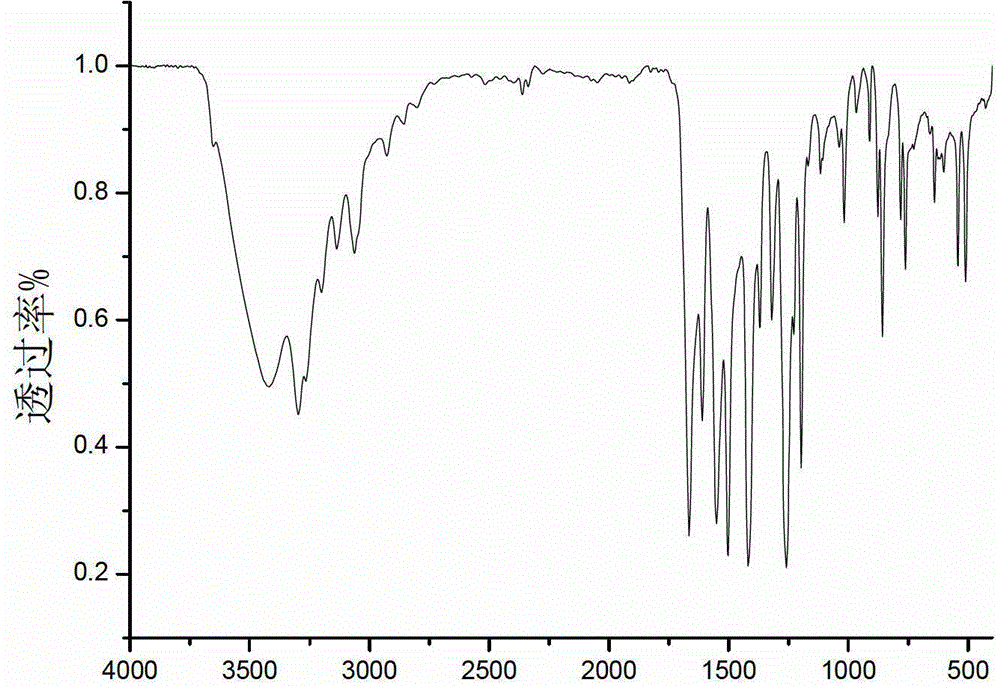
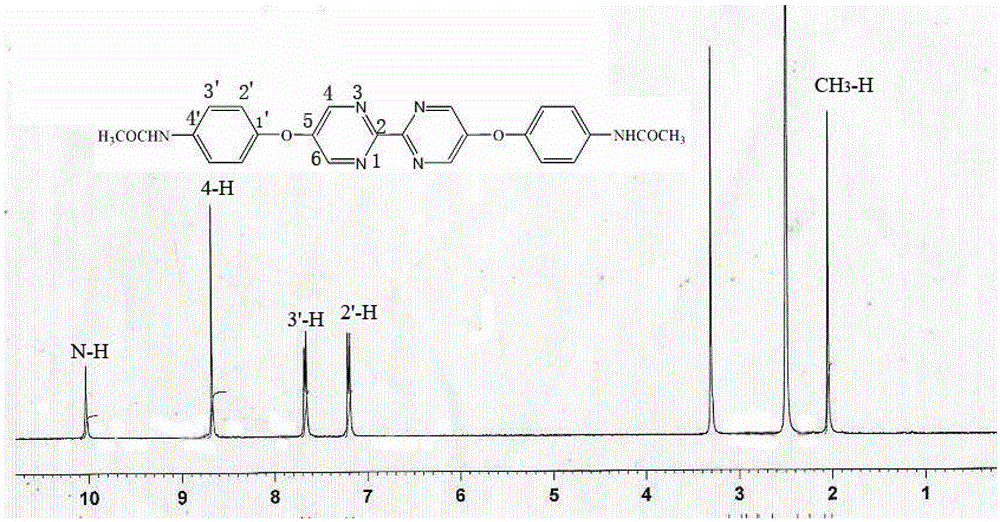
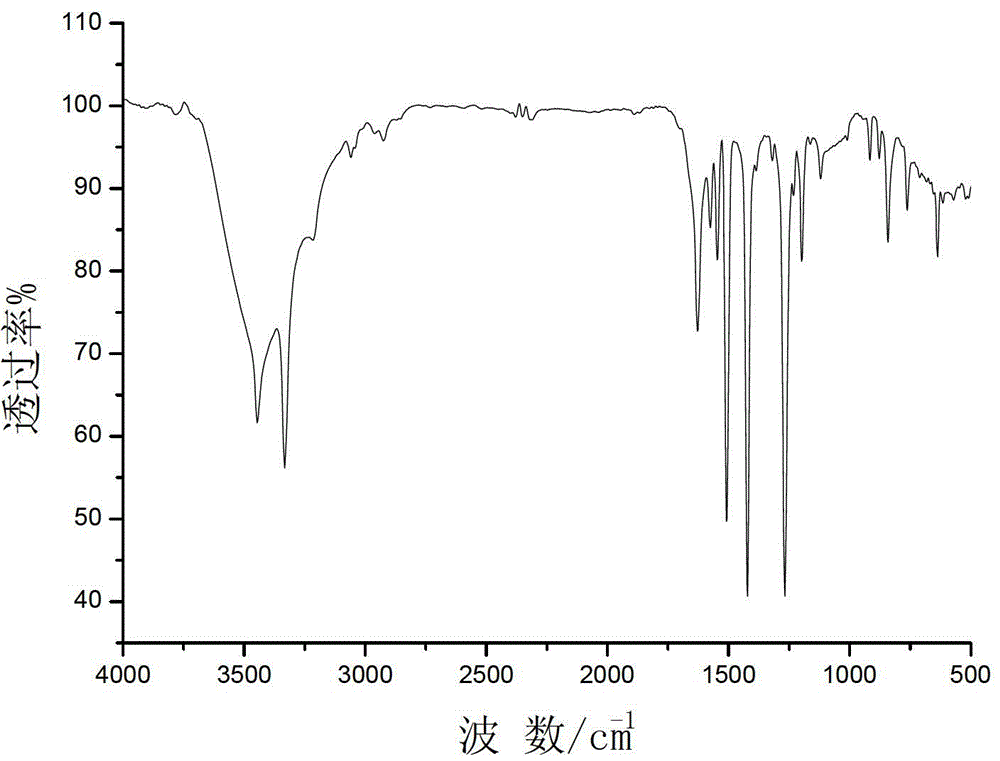
Bipyrimidyl dibenzene/diether/diamine and synthesis method thereof
A bipyrimidine and bipyrimidine-based technology, applied in the field of organic compounds and their preparation, can solve the problems of unfavorable production safety and environmental protection, low reaction yield, etc., to achieve safety production and environmental protection, improve reaction yield, The effect of performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 850ml of dehydrated N,N-dimethylformamide was deoxygenated with argon for 5 hours, 45.80g (0.18mol) of triphenylphosphine, NiCl 2 .H 2 O10.40 g (0.044 mol) and activated zinc powder 5.70 g (0.086 mo) were deoxygenated under vacuum for 20 minutes, added to DMF under the protection of argon, and stirred at room temperature under the protection of argon. After one hour, add 20.00 g (0.18 mol) of 2-chloropyrimidine, react at room temperature for one hour, then react at 50°C for 30 hours, filter with suction, wash the solid with chloroform, evaporate the filtrate to dryness, add 75 g of ETDA and NH to the solid 3 .H 2 O (200mL, 7%) was stirred in a solution, extracted with ethyl acetate (3×200mL), and the remaining solution was extracted with chloroform (8×150mL), washed with Na 2 SO 4 Dry and evaporate chloroform to dryness to obtain a light yellow solid, which is recrystallized from ethyl acetate:methanol=19:1 to obtain 23.87 g of white product bipyrimidine with a yield...
Embodiment 2
[0047] 850ml of dehydrated N,N-dimethylformamide was deoxygenated with argon for 5 hours, 45.80g (0.18mol) of triphenylphosphine, NiCl 2 .H 2 O10.40 g (0.044 mol) and activated zinc powder 5.70 g (0.086 mo) were deoxygenated under vacuum for 20 minutes, added to DMF under the protection of argon, and stirred at room temperature under the protection of argon. After one hour, add 20.00 g (0.18 mol) of 2-chloropyrimidine, react at room temperature for one hour, then react at 50°C for 30 hours, filter with suction, wash the solid with chloroform, evaporate the filtrate to dryness, add 75 g of ETDA and NH to the solid 3 .H 2 O (200mL, 7%) was stirred in a solution, extracted with ethyl acetate (3×200mL), and the remaining solution was extracted with chloroform (8×150mL), washed with Na 2 SO 4 Dry and evaporate chloroform to dryness to obtain a light yellow solid, which is recrystallized from ethyl acetate:methanol=19:1 to obtain 23.87 g of white product bipyrimidine with a yield...
Embodiment 3
[0054] 850ml of dehydrated N,N-methylformamide was deoxygenated with argon for 5 hours, 45.80g (0.18mol) of triphenylphosphine, NiCl 2 .H 2 O10.40 g (0.044 mol) and activated zinc powder 5.70 g (0.086 mo) were deoxygenated under vacuum for 20 minutes, added to DMF under the protection of argon, and stirred at room temperature under the protection of argon. After one hour, add 20.00 g (0.18 mol) of 2-chloropyrimidine, react at room temperature for one hour, then react at 50°C for 30 hours, filter with suction, wash the solid with chloroform, evaporate the filtrate to dryness, add 75 g of ETDA and NH to the solid 3 .H 2 O (200mL, 7%) was stirred in a solution, extracted with ethyl acetate (3×200mL), and the remaining solution was extracted with chloroform (8×150mL), washed with Na 2 SO 4 Dry and evaporate chloroform to dryness to obtain a light yellow solid, which is recrystallized from ethyl acetate:methanol=19:1 to obtain 23.87 g of white product bipyrimidine with a yield o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com