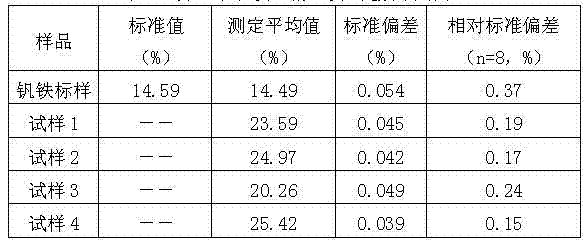
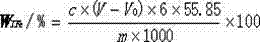Method for rapidly determining content of total iron in nitrided ferrovanadium
A rapid determination technology for ferrovanadium nitride, applied in the preparation of test samples, chemical analysis by titration, etc., can solve the problems of difficult to obtain accurate results, provide quality data, cumbersome operation, etc., and prevent molten samples from splashing , to ensure accuracy, and to simplify the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: The processing steps of the method for the rapid determination of total iron content in ferrovanadium nitride are as follows.
[0026] (1) Decomposition of the sample: Weigh 0.2000g of sample A into a high-alumina crucible filled with 3g of sodium peroxide in advance. Heat and melt in an electric furnace at 250°C. After all the solids in the crucible are melted, transfer to a muffle furnace at 700°C for melting for 10 minutes, take it out and cool slightly, and obtain an alkali fusion sample;
[0027] (2) Eliminate the interference of high vanadium on the determination of total iron: put the slightly cooled crucible above into a 400mL beaker filled with 20mL analytically pure concentrated hydrochloric acid (mass fraction 36-38%) in advance to extract the sample, wash out the crucible, Obtain leaching solution; Add 20mL, 50wt% sodium hydroxide in leaching solution, after solution precipitation is complete, filter with medium-speed quantitative filter paper,...
Embodiment 2
[0040] Embodiment 2: The process steps of the method for the rapid determination of total iron content in ferrovanadium nitride are as follows.
[0041] (1) Decomposition of the sample: Weigh 0.1500g of sample B into a high-alumina crucible containing 2g of sodium carbonate and sodium hydroxide (mass ratio 1:1) mixed flux in advance, mix well, and then cover with 1g of the above-mentioned Mix the flux, place it on a 300°C electric furnace and heat it to melt. After all the solids in the crucible have melted, transfer it to a 750°C muffle furnace to melt for 15 minutes, and take it out to cool slightly;
[0042](2) Eliminate the interference of high vanadium on the determination of total iron: put the slightly cooled crucible above into a 400mL beaker filled with 22.5mL analytically pure concentrated hydrochloric acid in advance to extract the sample, wash out the crucible, and add 18mL, 80wt% sodium hydroxide, after the solution precipitates completely, filter with medium-spee...
Embodiment 3
[0047] Embodiment 3: The processing steps of the method for the rapid determination of total iron content in ferrovanadium nitride are as follows.
[0048] (1) Decomposition of the sample: Weigh 0.2500g of sample C into a high-alumina crucible containing 7g of sodium carbonate and sodium peroxide (mass ratio 1:1) mixed flux in advance, mix well, and then cover with 3g of mixed flux For fluxing agent, place it on a 200°C electric furnace to heat and melt it. After all the solids in the crucible have melted, transfer it to an 800°C muffle furnace to melt for 12 minutes, and take it out to cool slightly;
[0049] (2) Eliminate the interference of high vanadium on the determination of total iron: put the slightly cooled crucible above into a 400mL beaker filled with 30mL of analytically pure concentrated hydrochloric acid in advance to extract the sample, wash out the crucible, and add 37.5mL, 20% sodium hydroxide, after the solution is completely precipitated, filter it with medi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com