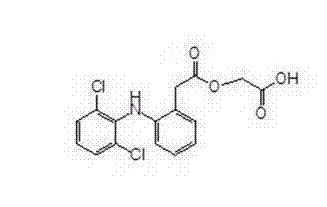
Aceclofenac preparation method
A technology of aceclofenac and acid hydrolysis, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of cyanide reactions, etc., can solve the problems of poor purity of crude products, difficulty in controlling the degree of acid hydrolysis, and difficulty in obtaining it. Achieve the effect of high selectivity, high conversion rate and easy refining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1: Preparation of tert-butyl 2-((2,6-dichlorophenyl)amino)phenylacetoxyacetate (tert-butyl aceclofenac)
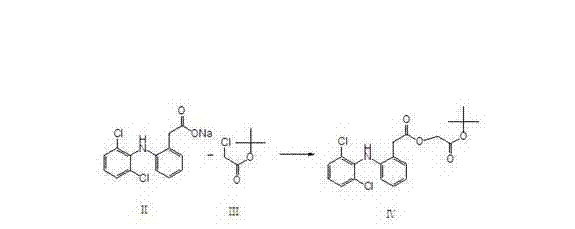
[0011] Dissolve diclofenac sodium shown in formula II and tert-butyl haloacetate shown in formula III, represented by tert-butyl chloroacetate in the following formula, in hydrocarbon solvents, ketone solvents or ether solvents, and add phase transfer Catalyst, carry out esterification reaction, obtain the aceclofenac tert-butyl ester shown in formula IV through aftertreatment.
[0012]
preparation example
[0013] The tert-butyl haloacetate used can be tert-butyl chloroacetate or tert-butyl bromoacetate, and the phase transfer catalyst used is a quaternary ammonium compound, which can be such as benzyltriethylamine halide, tetrabutylammonium halide, The hydrocarbon solvent used can be alkane or aromatic hydrocarbon or halocarbon, the ketone solvent can be acetone or butanone, etc., and the ether solvent refers to ether or methyl tert-butyl ether. The specific preparation examples are as follows:
[0014] 85L of toluene was pumped into the reaction tank, and 40Kg of diclofenac sodium, 20Kg of tert-butyl chloroacetate, and 0.1Kg of benzyltriethylamine chloride were added. The temperature was raised to reflux, and the reaction was carried out for 6 hours. Cool down to 75°C, add 50Kg of water, and stir for 20 minutes. Let stand for 20 minutes. Separate the aqueous layer. Toluene was recovered under reduced pressure to dryness. Add 60L methanol to dissolve. Slowly cool down to ro...
example 2
[0015] Example 2: Preparation of Aceclofenac
[0016] Put 75kg of formic acid into the reaction tank, add 40Kg of tert-butyl aceclofenac, stir to dissolve, and feed hydrogen chloride until the content (w / w) of hydrogen chloride is 6%. Control the temperature at 60°C, continue to stir and react for 5 hours, lower the temperature to 20°C, let stand for 3 hours until crystallization is complete, top wash, drain, and dry at 80°C to obtain 32.9 Kg of aceclofenac with a yield of 95.32%. The crude product was dissolved in 120L of toluene and heated to above 90°C. Filter and cool to room temperature. Until the crystallization is complete, filter, wash with a small amount of toluene, and drain. Obtain 31.4 Kg of aceclofenac fine works. The refined yield is 92%. HPLC content 99.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com