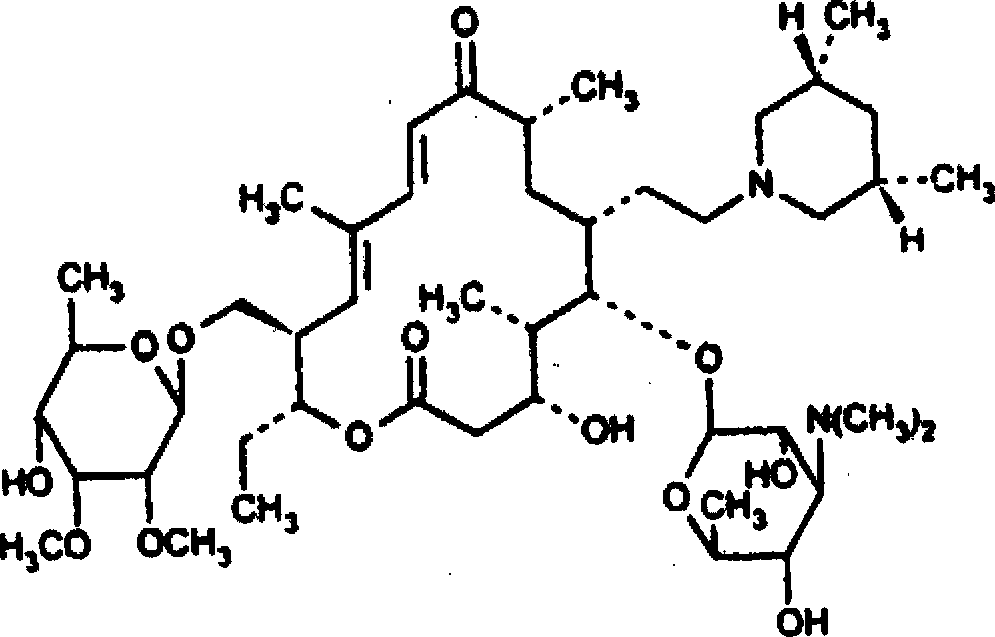
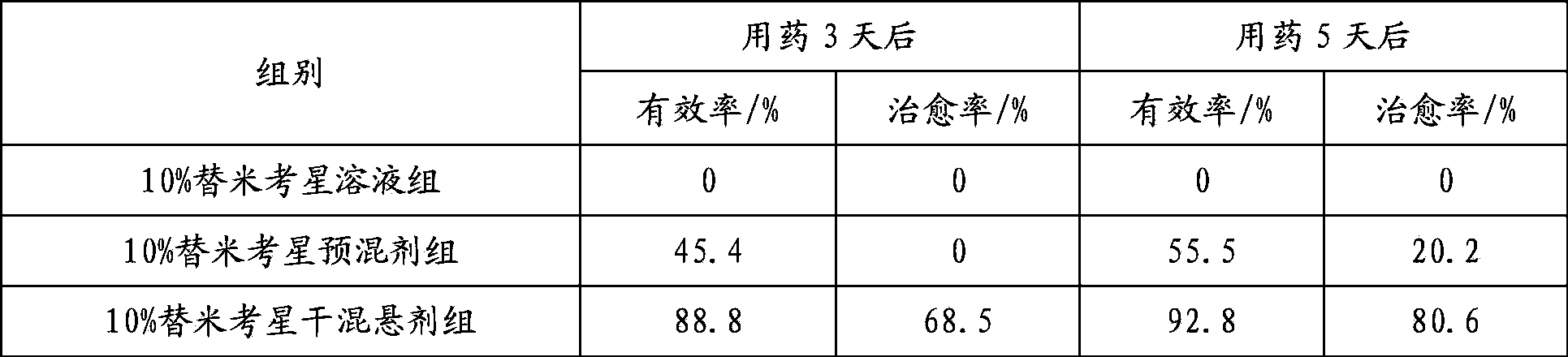
Tilmicosin dry suspension, method for preparing dry suspension and uses thereof
A technology of tilmicosin and dry suspension, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of preparing dry suspensions without micosin To achieve the effect of facilitating clinical drinking water administration, expanding the scope of application, and improving palatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 tilmicosin dry suspension of the present invention
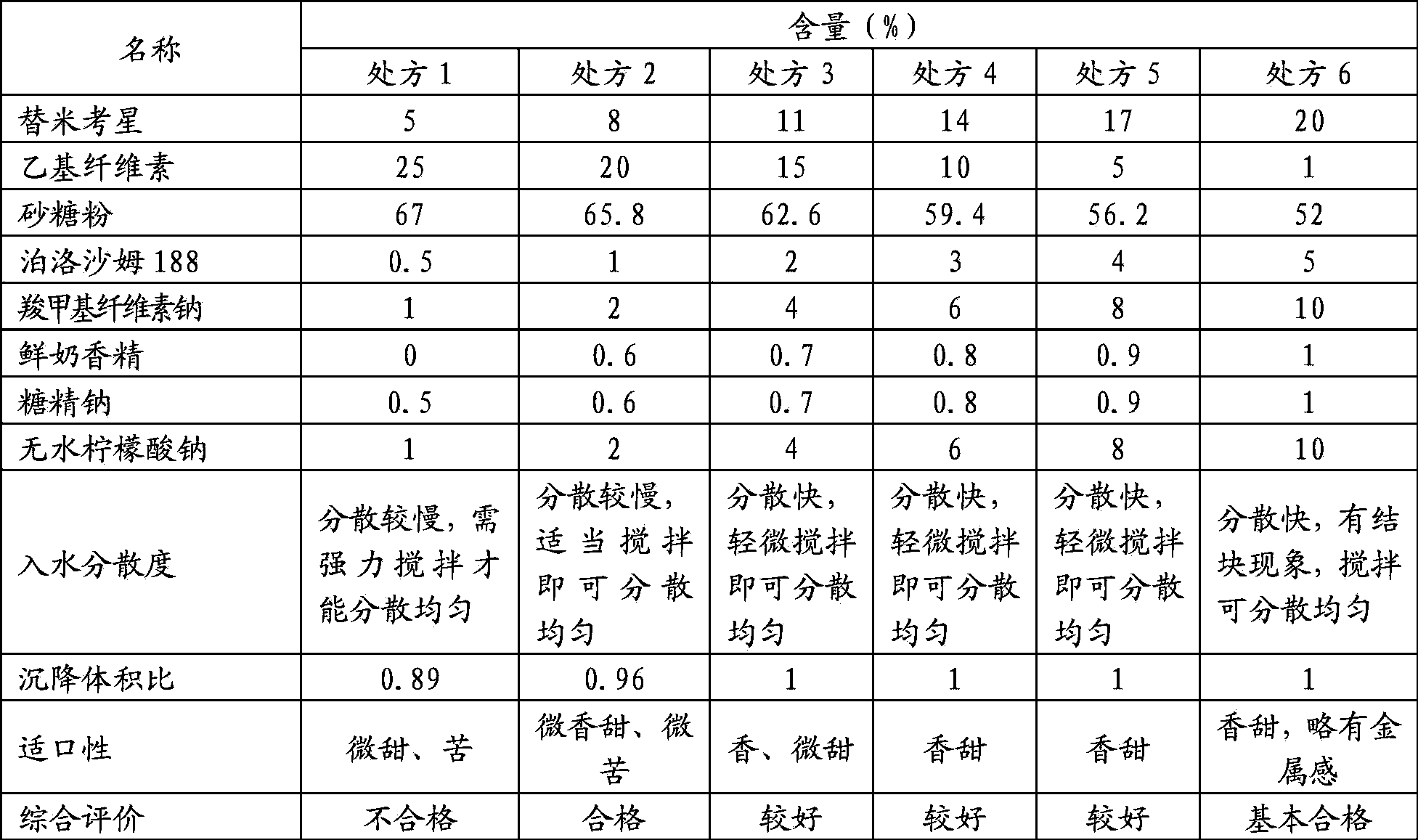
[0023] prescription:
[0024] Table 1 Prescription Ⅰ of tilmicosin dry suspension
[0025] name
Quantity / kg
5.75
25
powdered sugar
64
Poloxamer 188
2.5
Sodium carboxymethyl cellulose
1.25
0.5
1
total
100
[0026] Process: 1. Take white granulated sugar, crush it, pass through an 80-mesh sieve, and weigh the prescription amount for later use;
[0027] 2. Take anhydrous sodium citrate and sodium saccharin according to the prescription and mix them by hand, then add sodium carboxymethylcellulose and poloxamer 188 and mix them by hand, then add tilmicosin and ethyl cellulose and mix them evenly. Pulverize and pass through a 200-mesh sieve for subsequent use;
[0028] 3. Put the powder obtained in the above two s...
Embodiment 2
[0029] Embodiment 2 The preparation of tilmicosin dry suspension of the present invention
[0030] prescription:
[0031] Table 2 Tilmicosin dry suspension prescription Ⅱ
[0032] name
Quantity / kg
Tilmicosin
10
15
powdered sugar
66.8
Poloxamer 188
2.5
Sodium carboxymethyl cellulose
2.5
0.6
0.6
2
total
100
[0033] Process: 1. Take white granulated sugar, crush it, pass through an 80-mesh sieve, and weigh the prescription amount for later use;
[0034] 2. Take anhydrous sodium citrate and sodium saccharin according to the prescription and mix them by hand, then add sodium carboxymethylcellulose and poloxamer 188 and mix them by hand, then add tilmicosin and ethyl cellulose and mix them evenly. Pulverize and pass through a 200-mesh sieve for subsequent use;
[0035] 3. Put the powde...
Embodiment 3
[0036] Embodiment 3 The preparation of tilmicosin dry suspension of the present invention
[0037] prescription:
[0038] Table 3 Tilmicosin dry suspension prescription III
[0039] name
Quantity / kg
Tilmicosin
14
10
powdered sugar
59.4
Poloxamer 188
3
Sodium carboxymethyl cellulose
6
0.8
0.8
6
[0040] Process: 1. Take white granulated sugar, crush it, pass through an 80-mesh sieve, and weigh the prescription amount for later use;
[0041] 2. Take anhydrous sodium citrate and sodium saccharin according to the prescription and mix them by hand, then add sodium carboxymethylcellulose and poloxamer 188 and mix them by hand, then add tilmicosin and ethyl cellulose and mix them evenly. Pulverize and pass through a 200-mesh sieve for subsequent use;
[0042] 3. Put the powder obtained in the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com