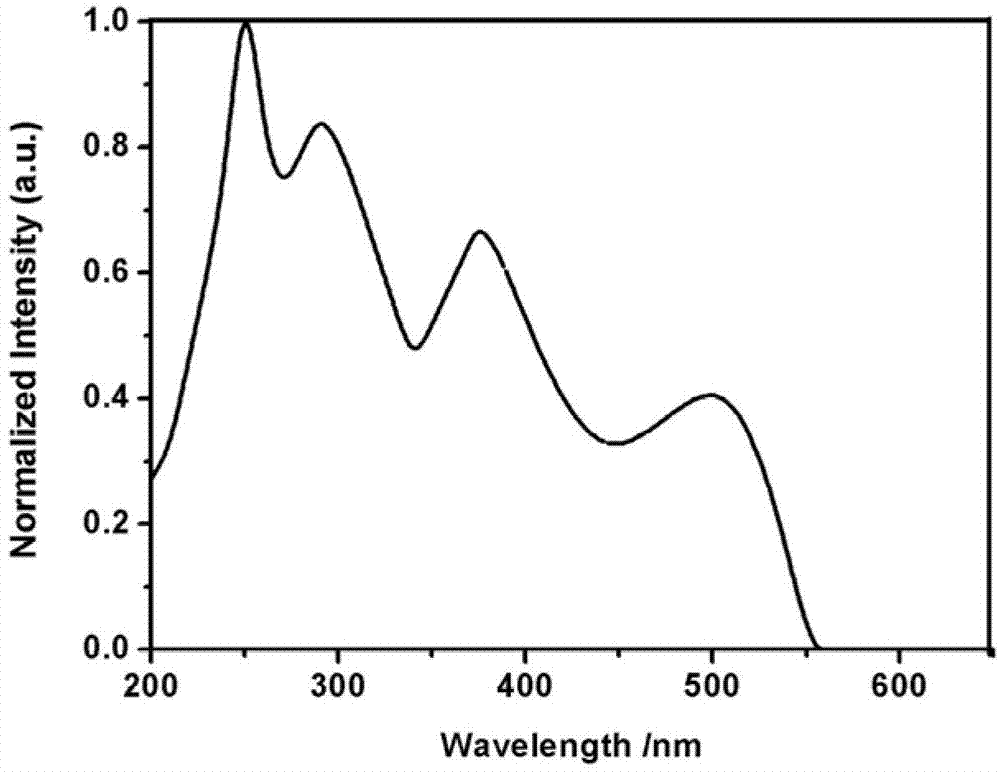
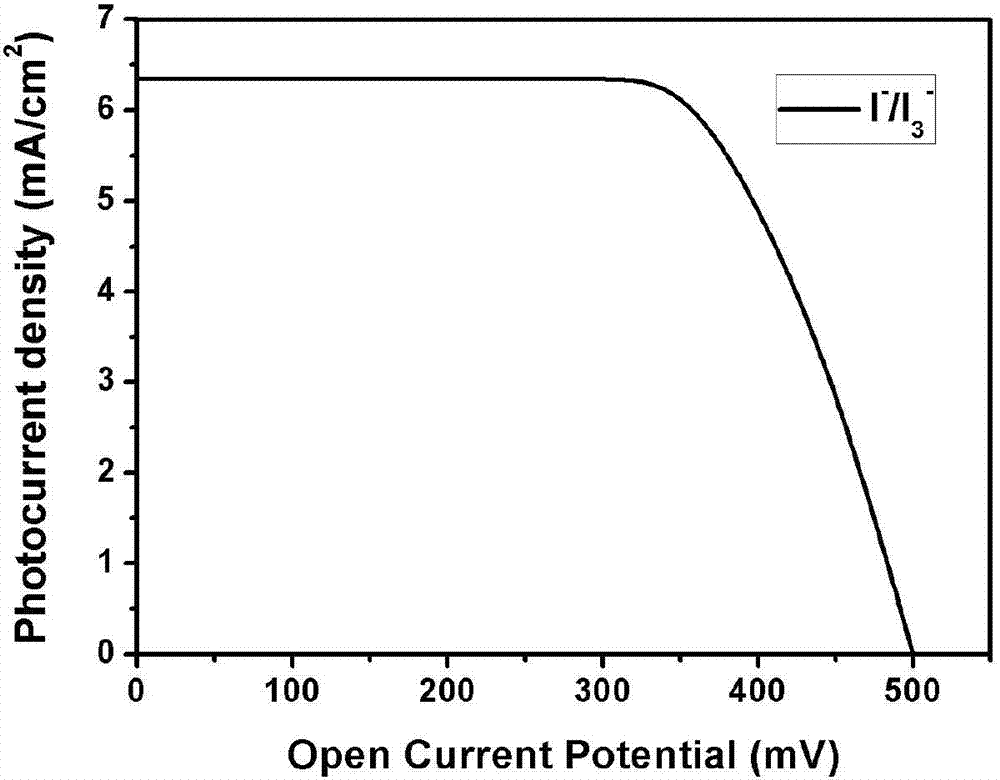
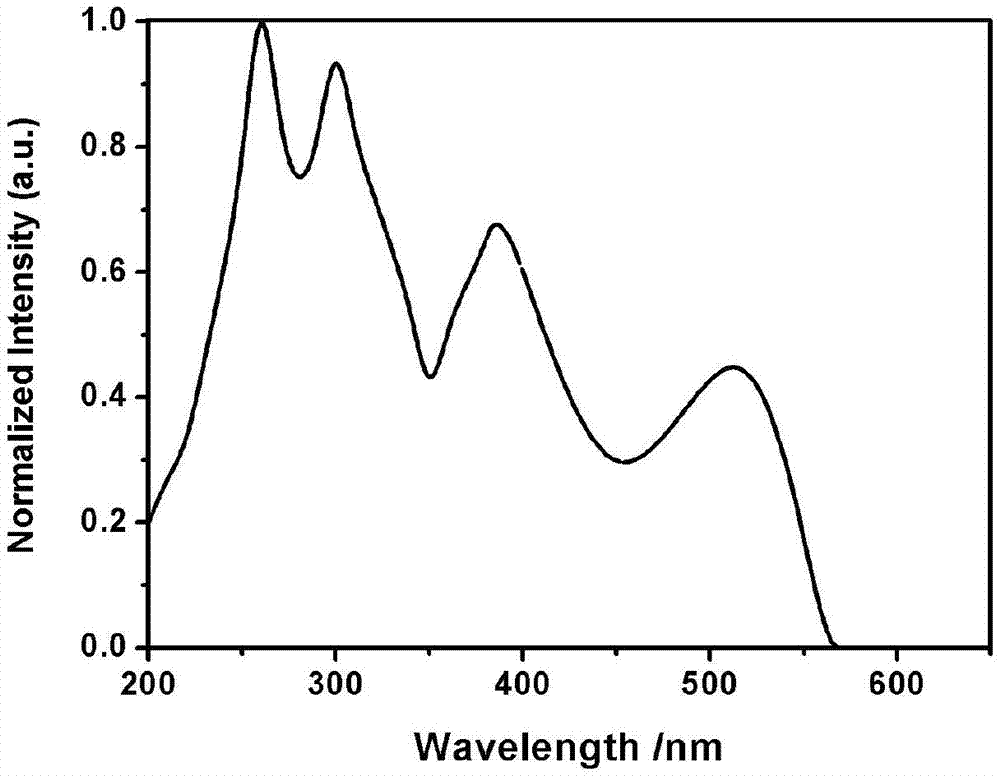
Pyrene D-pi-A type asymmetric disc dye compound
A dye compound, asymmetric technology, applied in the preparation of organic compounds, azo dyes, organic dyes, etc., can solve problems such as reduced efficiency, and achieve the effects of low cost, simple synthesis process and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] to:R 1 structured as R 2 structured as The synthetic method of this compound is introduced as an example
[0032] Bromination reaction of A pyrene: Dissolve 10mmol pyrene (2.02g) in 50mL nitrobenzene, put it in a 100mL single-necked bottle, gradually add 30mmol liquid bromine (2.00mL) dropwise at room temperature, after the dropwise addition, raise the temperature to To 100-120 degrees, stop the reaction after 16-24 hours of reaction. Pour the product into a solution containing 200mL of 10% sodium bisulfite and stir to remove the remaining bromine, change the water every ten minutes until the solution is colorless; separate the liquid to remove the water layer, add methanol to the organic layer to produce particles After filtration, washed with water and ethanol, dried to obtain 2.81 g of a yellow powder product, the yield was 78%.
[0033] B Sonogashira coupling reaction: Dissolve 5mmol of the product (1.80g) obtained in A in a mixed solution of 30mL tetrahydrof...
Embodiment 2
[0039] with R 1 structured as R 2 structured as The synthetic method of this compound is introduced as an example
[0040]Bromination reaction of A pyrene: Dissolve 10mmol pyrene (2.02g) in 50mL nitrobenzene, put it in a 100mL single-necked bottle, gradually add 30mmol liquid bromine (2.00mL) dropwise at room temperature, after the dropwise addition, raise the temperature to To 100-120 degrees, stop the reaction after 16-24 hours of reaction. Pour the product into a solution containing 200mL of 10% sodium bisulfite and stir to remove the remaining bromine, change the water every ten minutes until the solution is colorless; separate the liquid to remove the water layer, add methanol to the organic layer to produce particles After filtration, washed with water and ethanol, dried to obtain 2.81 g of a yellow powder product, the yield was 78%.
[0041] B Sonogashira coupling reaction: Dissolve 5mmol of the product (1.80g) obtained in A in a mixed solution of 30mL tetrahydro...
Embodiment 3
[0047] with R 1 structured as R 2 structured as The synthetic method of this compound is introduced as an example
[0048] Bromination reaction of A pyrene: Dissolve 10mmol pyrene (2.02g) in 50mL nitrobenzene, put it in a 100mL single-necked bottle, gradually add 30mmol liquid bromine (2.00mL) dropwise at room temperature, after the dropwise addition, raise the temperature to To 100-120 degrees, stop the reaction after 16-24 hours of reaction. Pour the product into a solution containing 200mL of 10% sodium bisulfite and stir to remove the remaining bromine, change the water every ten minutes until the solution is colorless; separate the liquid to remove the water layer, add methanol to the organic layer to produce particles After filtration, washed with water and ethanol, dried to obtain 2.81 g of a yellow powder product, the yield was 78%.
[0049] B Sonogashira coupling reaction: Dissolve 5mmol of the product (1.80g) obtained in A in a mixed solution of 30mL tetrahydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com