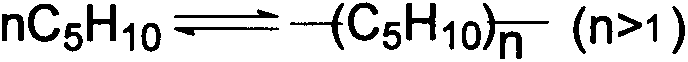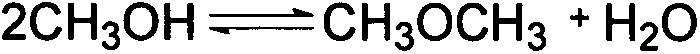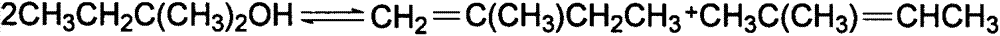Catalyst for preparing isoamylene by splitting decomposition of t-amyl-methyl ether and preparation method and application thereof
A technology of methyl tert-amyl ether and isopentene, which is applied in chemical instruments and methods, hydroxyl compound preparation, physical/chemical process catalysts, etc., can solve the problem of high energy consumption, low conversion rate and low space velocity of ether hydrolysis And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]30 g γ-Al 2 o 3 Add it to 50ml of 20% phosphoric acid solution and let stand at room temperature for 1h, then filter, dry at 90°C for 4h, and roast at 800°C for 4h to obtain PO 4 = / Al 2 o 3 .
[0031] The product was then dissolved in 40ml of 18% La(NO 3 ) 3 In aqueous solution, soak for 6 hours, filter, dry at 100°C for 8 hours, and roast at 800°C for 8 hours to obtain La 2 o 3 / PO 4 = / Al 2 o 3 .
[0032] La 2 o 3 / PO 4 = / Al 2 o 3 Add it into 50ml of 20% ammonium fluoride solution, filter, dry at 100°C for 4h, and roast at 800°C for 5h to obtain F / La of the present invention 2 o 3 / PO 4 = / Al 2 o 3 .
[0033] Put 14g of catalyst into a miniature fixed-bed reactor with a diameter of Φ20mm, feed methyl tert-amyl ether with a liquid micro-sampling pump, and control the liquid space velocity to 2h -1 , the reaction temperature is 170°C, the conversion rate of TAME is 99.4%, the selectivity of isoamylene is 100%, and the selectivity of methanol i...
Embodiment 2
[0035] 30 g γ-Al 2 o 3 Add it to 50ml of 46% ammonium bisulfate solution and let stand at room temperature for 8h, then filter, dry at 100°C for 4h, and roast at 800°C for 3h to obtain SO 4 = / Al 2 o 3 .
[0036] The product was then dissolved in 40ml of 0.5% Ce(NO 3 ) 3 In aqueous solution, soak for 2h, filter, dry at 150°C for 1h, and calcinate at 800°C for 4h to obtain Ce 2 o 3 / SO 4 = / Al 2 o 3 .
[0037] Then Ce 2 o 3 / SO 4 = / Al 2 o 3 Add it to 50ml of 30% sodium fluoride solution, filter, dry at 120°C for 5h, and roast at 800°C for 5h to obtain the F / Ce of the present invention 2 o 3 / SO 4 = / Al 2 o 3 .
[0038] Put 14g of catalyst into a miniature fixed-bed reactor with a diameter of Φ20mm, feed methyl tert-amyl ether with a liquid micro-sampling pump, and control the liquid space velocity to 3h -1 , the reaction temperature is 160°C, the conversion rate of TAME is 99.6%, the selectivity of isoamylene is 100%, and the selectivity of methanol i...
Embodiment 3
[0040] 30 g γ-Al 2 o 3 Add it to 50ml of 0.5% ammonium phosphate solution and let stand at room temperature for 6h, then filter, dry at 100°C for 8h, and roast at 300°C for 8h to obtain PO 4 = / Al 2 o 3 .
[0041] The product was then dissolved in 40ml of 10% Sm(NO 3 ) 3 Soak in aqueous solution for 6h, filter, dry at 100°C for 4h, and roast at 800°C for 1h to obtain Sm 2 o 3 / PO 4 = / Al 2 o 3 .
[0042] Sm again 2 o 3 / PO 4 = / Al 2 o 3 Add it to 50ml of 40% hydrofluoric acid solution and soak for 1h, filter, dry at 100°C for 4h, and roast at 800°C for 5h to obtain the F / Sm of the present invention 2 o 3 / PO 4 = / Al 2 o 3 .
[0043] Put 14g of catalyst into a miniature fixed-bed reactor with a diameter of Φ20mm, feed methyl tert-amyl ether with a liquid micro-sampling pump, and control the liquid space velocity to 2h -1 , the reaction temperature is 170°C, the conversion rate of TAME is 99.1%, the selectivity of isoamylene is 100%, and the selectivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com