Application of hydrogen sulfide releasing agent in preparation of medicament for treating renal fibrosis disease
A release agent, hydrogen sulfide technology, applied in drug combinations, urinary system diseases, active ingredients of sulfur/selenium/tellurium, etc., can solve problems such as deterioration, exacerbation of renal function deterioration, and elevated serum creatinine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 MTT method for measuring the proliferation of renal interstitial fibroblasts
[0069] Renal interstitial fibroblasts were placed in DMEM medium containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured in an incubator. The medium was changed every other day, digested with 0.25% trypsin, and passaged at a ratio of 1:3 to obtain renal interstitial fibroblasts (NRK-49F).
[0070] Take 2×10 3 Renal interstitial fibroblasts (NRK-49F) were seeded in a 96-well plate and cultured. Serum (FBS) was used to simulate the effects of growth factors such as EGF and PDGF. Group experimental cells according to FBS concentration gradient: 1%FBS, 3%FBS, 5%FBS, 10%FBS, 1%FBS+Na 2 S, 3%FBS+Na 2 S, 5%FBS+Na 2 S, 10%FBS+Na 2 S, where Na 2 The concentration of S was 100 μM. Na 2 S pretreatment for 30 minutes. After 24 hours of cell treatment, 20 μL of MTT (5 mg / mL) was added to each well, the medium was discarded after 4 hours, 150 μL of DMSO was added to each well to di...
Embodiment 2
[0072] Example 2 MTT method for measuring the proliferation of renal interstitial fibroblasts
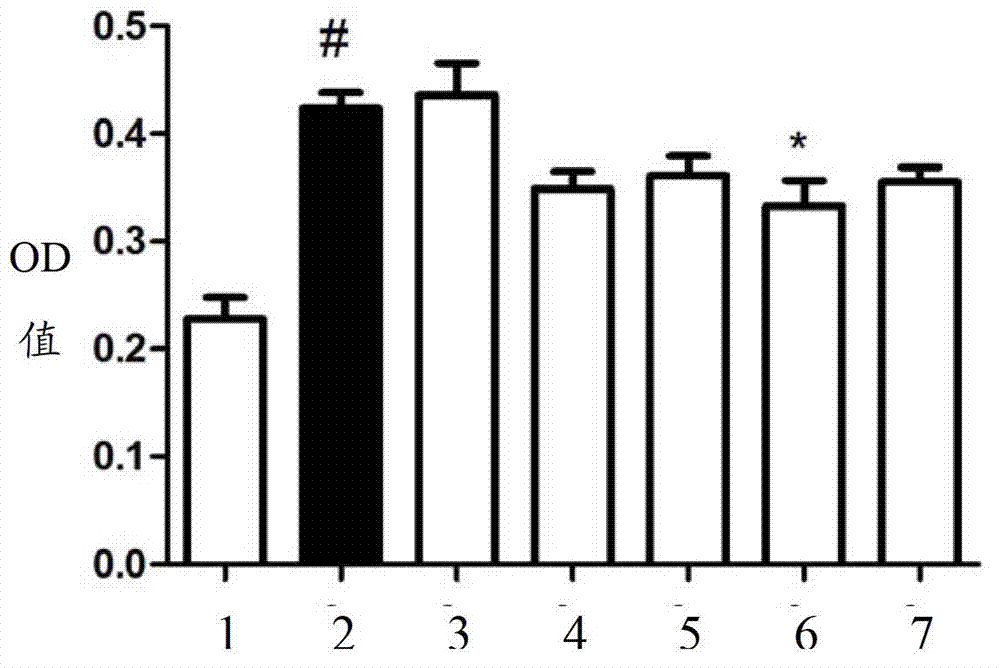
[0073] Take the 2 × 10 prepared in Example 1 3 Renal interstitial fibroblasts (NRK-49F) were seeded in a 96-well plate and cultured. Serum (FBS) was used to simulate the effects of growth factors such as EGF and PDGF. According to Na 2 S concentration gradient for experimental cell grouping: blank control group, FBS group, 1 μM Na 2 S+FBS, 10 μM Na 2 S+FBS, 50 μM Na 2 S+FBS, 100 μM Na 2 S+FBS, 500 μM Na 2 S+FBS, where FBS uses 10% FBS. Na 2 S pretreatment for 30 minutes. After 24 hours of cell treatment, 20 μL of MTT (5 mg / mL) was added to each well, the medium was discarded after 4 hours, 150 μL of DMSO was added to each well to dissolve formazan, and the OD value was read with a microplate reader at a wavelength of 490 nm. For test results, see figure 2 .
[0074] Depend on figure 2 It can be seen that Na 2 When the S concentration was 10-100 μM, the antiproliferat...
Embodiment 3
[0075] Example 3 BrdU infiltration method to measure the proliferation of renal interstitial fibroblasts
[0076] Take the 4 × 10 prepared in Example 1 4 Cells (NRK-49F) were seeded in 24-well plates and cultured overnight in DMEM medium containing 0.5% fetal calf serum. Experimental grouping after cell attachment: blank control group, 10% FBS group, Na 2 Group S (100μM), 10%FBS+Na 2 S (100 μM) group. Cell proliferation was stimulated by 10% FBS for 5 hours, and Brdu (10 μM) was added to continue incubation for 5 hours. After the cells were treated, they were fixed with 4% paraformaldehyde for 20 minutes, followed by 2N HCL DNA denaturation at 37°C for 20 minutes. After blocking with 5% BSA for 1 hour, anti-Brdu primary antibody (1:100) was added overnight at 4°C, Alexa Fluor555 Donkey Anti-Mouse IgG secondary antibody (1:500) was added at room temperature for 1 hour, and the slides were mounted using Vector mounting media containing DAPI. Observe 10 non-overlapping field...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com