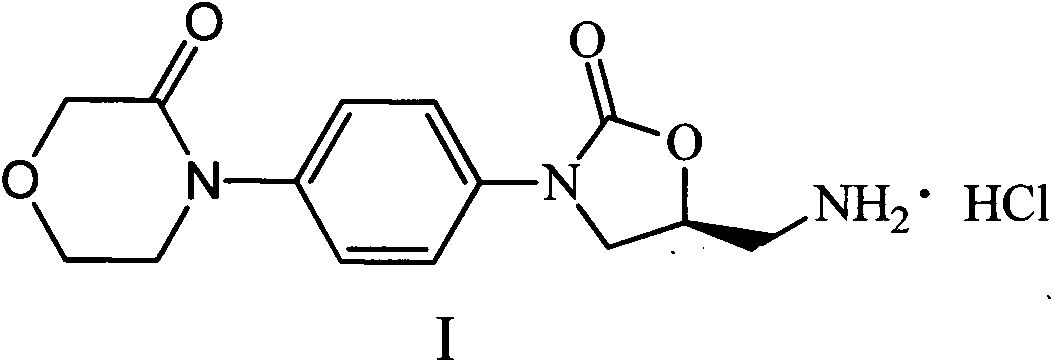
Rivaroxaban intermediate preparation method
A technology for rivaroxaban and intermediates, which is applied in the field of preparation of rivaroxaban intermediates, can solve the problems of unfavorable industrial production, difficulty in separation and purification, and low yield, and achieve favorable industrial production, easy availability of raw materials, The effect of high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
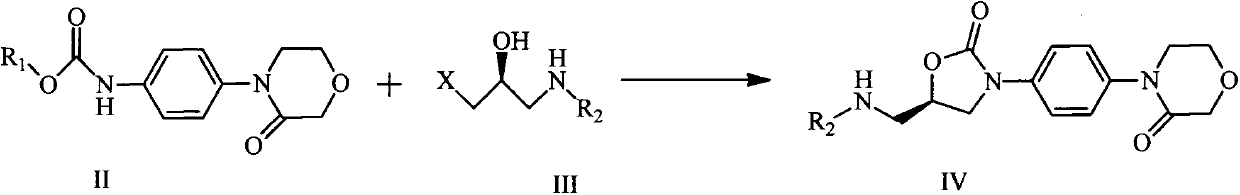
[0032] The preparation of compound IIa (compound IIa is R in compound II 1 benzyl compound)
[0033]
[0034] Add 19.2g of 4-(4-aminophenyl)-3-morpholinone and 12.6g of sodium bicarbonate into the reaction flask, add 200ml of water and 100ml of 1,4-dioxane, and add 20.4g of chloroformic acid dropwise at 0°C The benzyl ester was dropped within 30 minutes, continued to stir at 0°C for 1 hour, raised the temperature to 30°C and stirred for 2 hours, and filtered with suction to obtain 31.1 g of a white solid with a melting point of 200-205°C and a yield of 95.4%.
Embodiment 2
[0036] The preparation of compound IVa (compound IIIa is that X is chlorine in compound III, R 2 for tert-butoxycarbonyl compounds)
[0037]
[0038] Add 10.0g of compound IIa and 14.1g of compound IIIa into the reaction flask, add 10ml of anhydrous N,N-dimethylformamide, add dropwise 10.3g of sodium tert-butoxide dissolved in 40ml of anhydrous tetrahydrofuran at 0°C for 1 hour After the internal drop is completed, continue stirring at 0°C for 2 hours, raise the temperature to 30°C, stir for 25 hours, add saturated ammonium chloride, extract with dichloromethane to obtain an organic layer, and concentrate to obtain 10.5 g of off-white solid, melting point 171-174°C , yield 87.5%.
Embodiment 3
[0040] The preparation of compound IVa (compound IIIa is that X is chlorine in compound III, R 2 for tert-butoxycarbonyl compounds)
[0041]
[0042] Add 30.0g of compound IIa and 34.5g of compound IIIa into the reaction flask, add 75ml of anhydrous N,N-dimethylformamide, add dropwise 26.4g of sodium tert-butoxide dissolved in 150ml of anhydrous tetrahydrofuran at 0°C for 1 hour After the internal drop is completed, continue stirring at 0°C for 2 hours, raise the temperature to 50°C, stir for 20 hours, add saturated ammonium chloride, extract with dichloromethane to obtain an organic layer, and concentrate to obtain 33.5 g of off-white solid, melting point 171-174°C , yield 93.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com