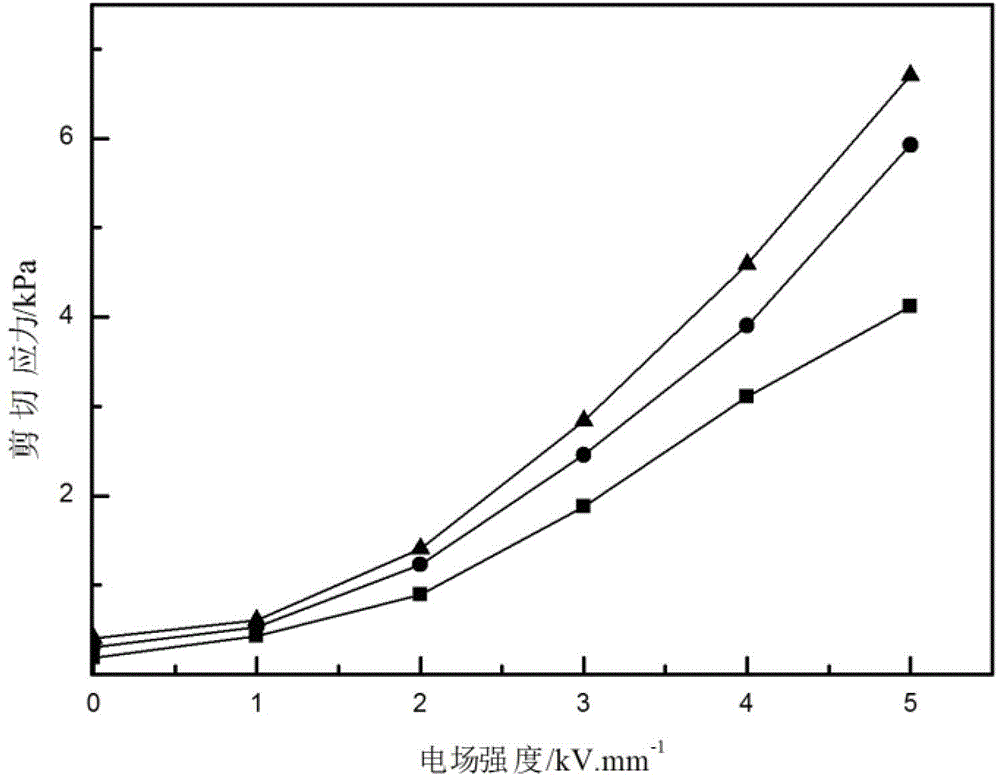
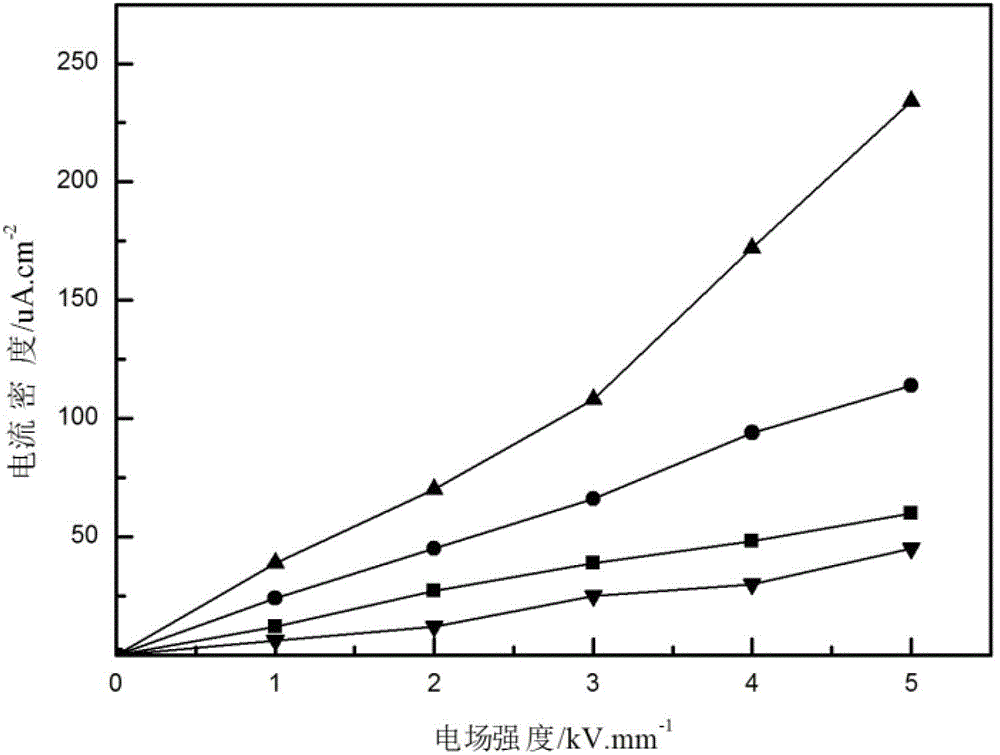
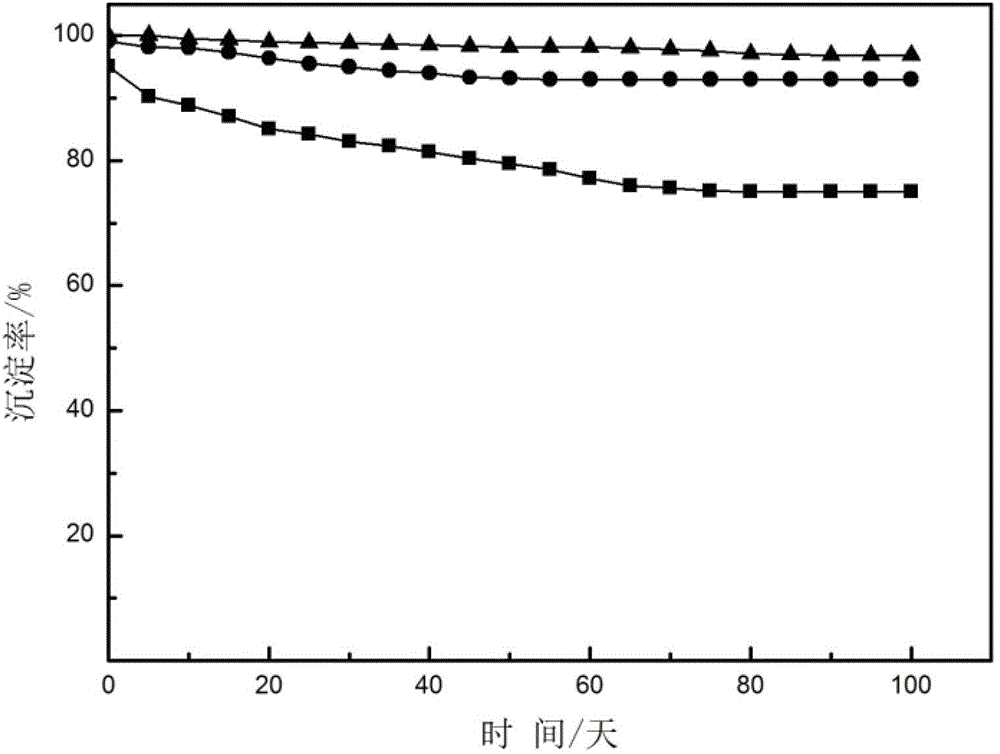
Preparation method of iron oxide particle
A technology of iron oxide and iron oxyhydroxide, applied in the field of electrorheological fluid, which can solve the problems of poor anti-precipitation performance of dispersed particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The embodiment of the present invention discloses a method for preparing iron oxide particles, comprising the following steps:
[0034] a) Mix iron salt, basic compound and dispersant in a solvent, react to obtain ferric oxyhydroxide, and coat the ferric oxyhydroxide with silicon dioxide;
[0035] b) heat-treating the product obtained in step a);
[0036] c) ultrasonically treating the product obtained in step b) in a basic compound to obtain iron oxide particles.
[0037] According to the present invention, in the process of preparing iron oxide particles, first react iron salt, basic compound and dispersant to obtain nanometer ferric oxyhydroxide; then nanometer ferric oxyhydroxide is coated with silicon dioxide, and then heat treated , to dehydroxylate ferric oxyhydroxide to obtain iron oxide coated with silica, and finally ultrasonically treat the iron oxide coated with silica in an alkaline compound to remove the oxide coated on the outer layer of iron oxide parti...
Embodiment 1
[0051] Adopt electronic analytical balance to weigh 20.2012gFe(NO 3 ) 3 9H 2 O and 3.0007g polyethylene glycol 400, dissolved in 250ml deionized water, heated and stirred with a magnetic stirrer at 140°C, kept the temperature of the reaction system at 100°C, heated for 2~3h, then slowly added 1M NaOH solution dropwise until The pH of the system is 9~10 to obtain ferric oxyhydroxide; then the ferric oxyhydroxide is centrifuged, washed 2 times with deionized water, and then washed 2~3 times with absolute ethanol to remove the water in the system, and then The washed particles are ultrasonically dispersed with a mixed solution of 100ml of ethanol and 400ml of cyclohexane, and then add 6ml of silane coupling agent and 15ml of 30wt% ammonia solution to the system. Ethyl silicate (TEOS) was reacted at room temperature for 24 hours to obtain ferric oxyhydroxide coated with silica. The obtained core-shell particles were washed with butanol, isopropanol, and ethanol in sequence, and ...
Embodiment 2
[0054] Adopt electronic analytical balance to weigh 20.2032g Fe(NO 3 ) 3 9H 2 O and 3.0022g polyethylene glycol 400, dissolved in 250ml deionized water, heated and stirred with a magnetic stirrer at 140°C, kept the temperature of the reaction system at 100°C, heated for 2~3h, then slowly added 1M NaOH solution dropwise until the system The pH is 9~10, and the ferric oxyhydroxide solution is obtained; the ferric oxyhydroxide solution is centrifuged, washed twice with deionized water, and then washed 2~3 times with absolute ethanol to remove the water in the system, and then The washed particles are ultrasonically dispersed with a mixed solution of 400ml cyclohexane and 100ml ethanol, then 6ml of silane coupling agent and 15ml of 30wt% ammonia solution are added to the system. Ethyl ester (TEOS) was reacted at room temperature for 24 hours to obtain ferric oxyhydroxide coated with silica; the obtained core-shell particles were washed with butanol, isopropanol and ethanol in se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com