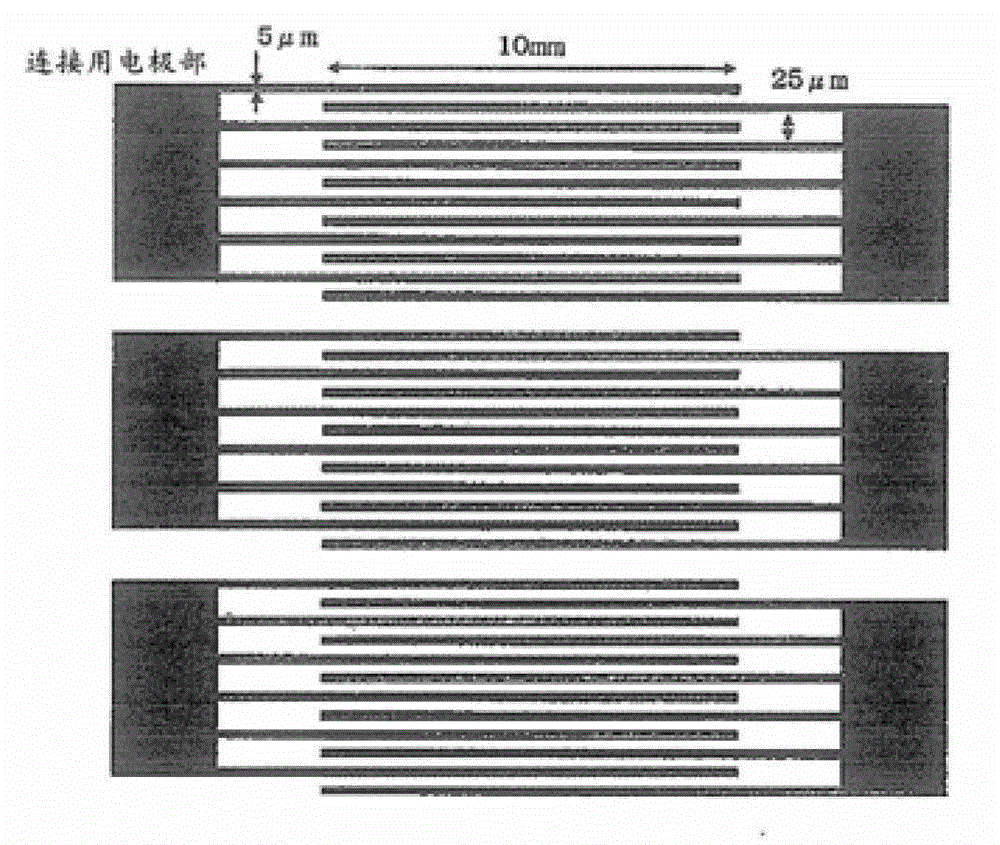
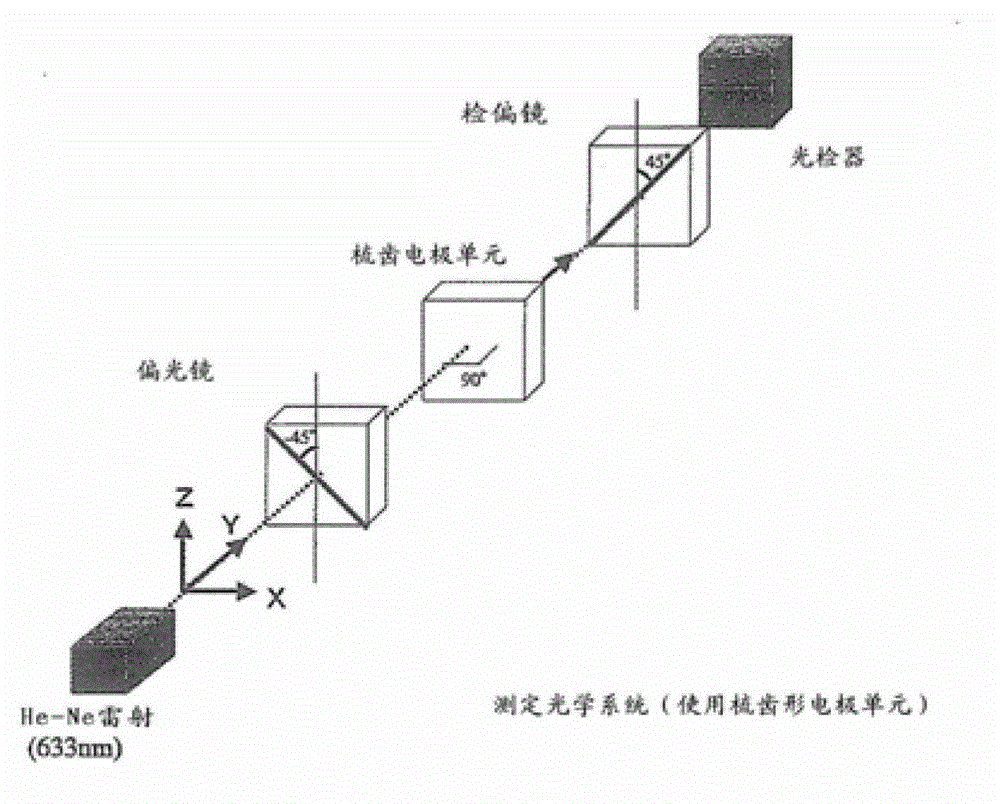
Compound having branched alkyl or branched alkenyl, optically isotropic liquid crystal medium and optical element
An isotropic, compound technology, applied in organic chemistry, optics, liquid crystal materials, etc., which can solve problems such as low stability, deterioration of voltage retention, and low chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0332] The liquid crystal composition of the present invention is usually prepared by a known method, for example, a method of dissolving essential components at high temperature.
[0333] 3 Compound (7)~Compound (11)
[0334] The third aspect of the present invention is a liquid crystal composition obtained by adding a component selected from component E and component F shown below to component A.
[0335] The component added to component A is preferably a component obtained by mixing the following components: at least 1 selected from the group consisting of the above formula (7), formula (8), formula (9) and formula (10). Component E comprising one compound or component F comprising at least one compound selected from the group consisting of the above formula (11).
[0336] In addition, even if each component of the liquid crystal composition used in the present invention is an analog containing an isotope of each element, there is no significant difference in physical prop...
Embodiment 1
[0481] Synthesis of compound (S1)
[0482]
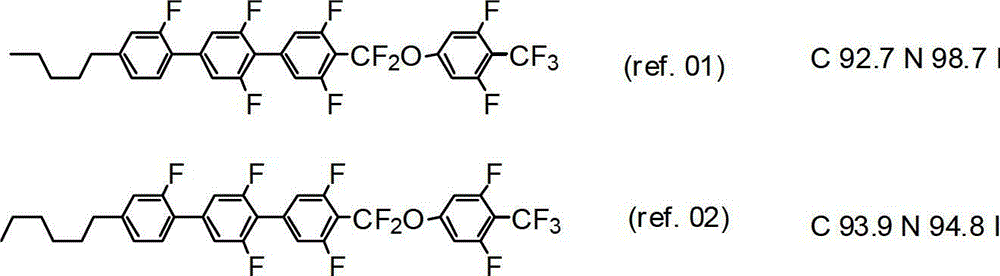
[0483] (In formula (1-1-2i), R 1a for C 4 h 9 , R 1b is hydrogen, L 1 is hydrogen, L 2 , L 3 and L 4 is fluorine, Y 1 for-CF 3 compound of. )
[0484] First, the synthetic schemes of compound (S1-03) and compound (S1-07) as intermediate raw materials are listed below.
[0485]
[0486] (Paragraph 1-1) Synthesis of compound (S1-03)
[0487] Under nitrogen flow, 1-bromo-3-fluoro-4-iodobenzene (S1-01) (21.5g, 71.6mmol), 3,5-difluoro-phenylboronic acid (S1-02) (11.3g, 71.6 mmol), dichloro(bistriphenylphosphine) palladium (0.503g, 0.716mmol), triphenylphosphine (0.375g, 1.43mmol), potassium carbonate (14.8g, 107mmol), tetrabutylammonium bromide (5.71 g, 17.9mmol), ethanol (100mL) and toluene (100mL) mixed solution, heated and stirred at 80°C for 6 hours. The reaction solution was poured into water, extracted twice with toluene (300 mL), washed with water three times, and the organic phase was...
Embodiment 2
[0514] Synthesis of compound (S2)
[0515]
[0516] (In formula (1-1-2i), R 1a for C 4 h 9 , R 1b for CH 3 , L 1 is hydrogen, L 2 , L 3 and L 4 is fluorine, Y 1 for-CF 3 compound of. )
[0517] 1-Bromo-3-ethylheptane (S2-07) is commercially available. The synthetic scheme of compound (S2) is listed.
[0518]
[0519] (Paragraph 2-1) Synthesis of compound (S2-08)
[0520] Compound (S2-08) (4.64 g) was obtained by performing the same operation as in Example 1 (paragraphs 1-4), using compound (S2-07) (5.04 g, 26.1 mmol), and performing the same operation , 14.5 mmol, yield: 55%).
[0521] (Paragraph 2-2) Synthesis of compound (S2)
[0522] Compound (S2) was obtained from compound (S2-08) (4.64 g, 14.5 mmol) obtained in the preceding paragraph by performing the same operation as in Example 1 (paragraphs 1-5) to (paragraphs 1-8) (1.20 g, 1.77 mmol, overall yield: 12%). The phase transition temperature (°C) of this compound is C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com