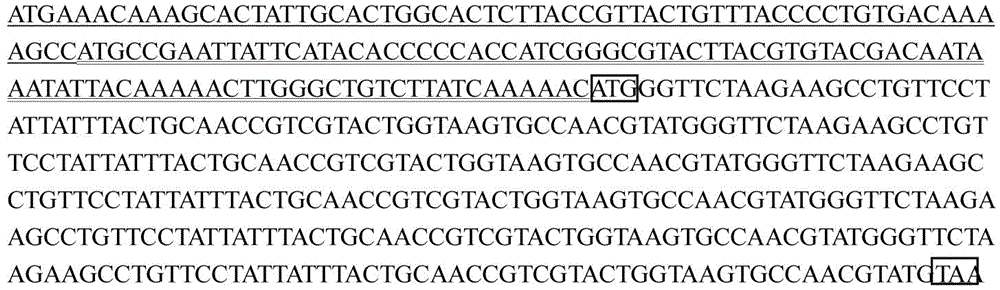Method for preparing thanatin based on escherichia coli prokaryotic expression system
A prokaryotic expression and death factor technology, applied in the field of genetic engineering, can solve the problems of host microorganism suicide, low yield, and inability to obtain expression products, and achieve the effect of avoiding toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] Experimental example 1 Construction of recombinant plasmid
[0023] Chemically synthesized recombinant gene, including sequentially linked guide peptide sequence, 90-base sequence of polyhedrin gene starting from the start codon, methionine codon, deadin repeat sequence and stop codon; deadin repeat sequence It is composed of 5 mortalin genes in series, and the mortalin gene sequence is deduced from the amino acid sequence of mortalin. See SEQ ID NO:1 for the sequence of the recombinant gene.
[0024] Load the recombinant gene into an expression vector to obtain a recombinant plasmid. Sanger sequencing was used to verify the correctness of the recombinant gene sequence.
[0025] It should be clearly stated that there are many ways to construct prokaryotic expression plasmids, which are easily done by those skilled in the art. Chemically synthesizing recombinant genes and loading them into expression vectors is only one of them; in addition, 5 methods are used here. T...
experiment example 2
[0027] Experimental example 2 Induced recombinant protein expression and SDS-PAGE detection
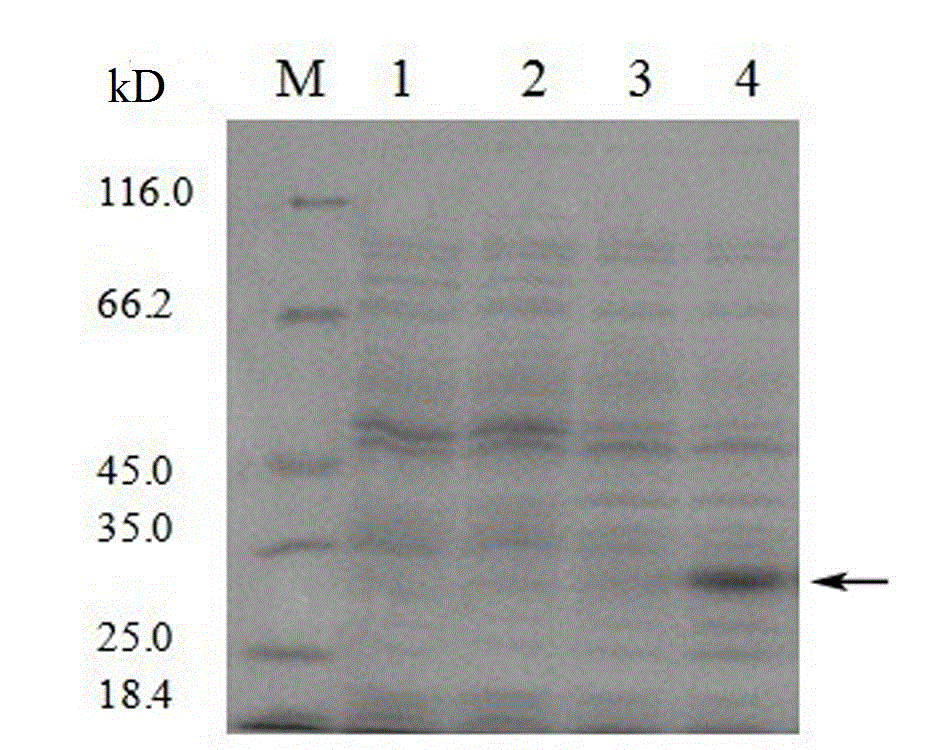
[0028] Escherichia coli containing the recombinant plasmid was induced to express the recombinant protein with IPTG, the recombinant protein was isolated and purified, and the size of the recombinant protein was detected by SDS-PAGE. Such as figure 1 As shown, after induction, recombinant protein expression is very obvious. The isolated and purified recombinant protein does not include the guide peptide, which is excised in Escherichia coli after completing the guide function. See SEQ ID NO: 2 for the complete amino acid sequence of the recombinant protein including the guide peptide.
[0029]
experiment example 3
[0030] Experimental example 3 Treatment of recombinant protein with cyanogen bromide
[0031] The recombinant protein obtained in Experimental Example 2 was treated with cyanogen bromide, and the recombinant protein was cut into multiple independent and complete deadin; the deadin was separated and purified.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com