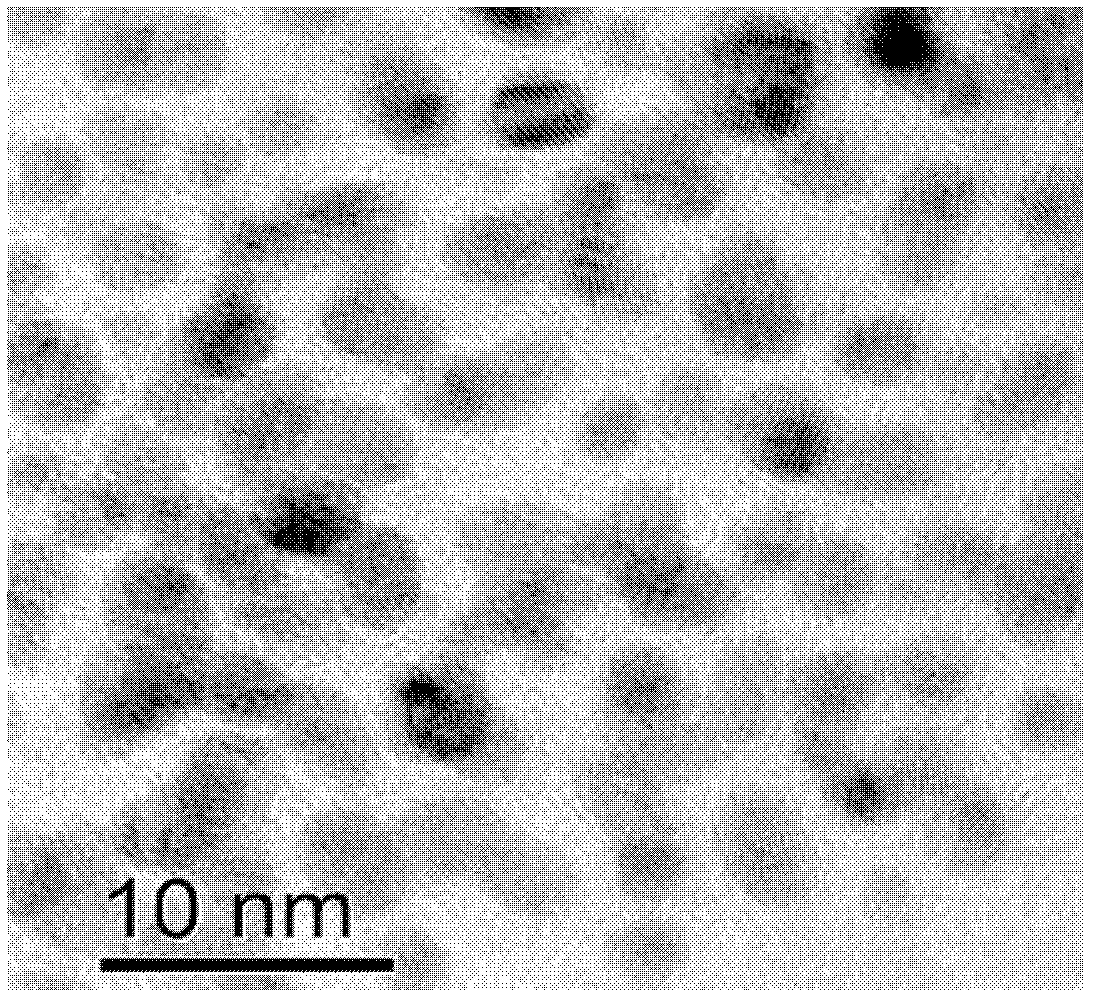
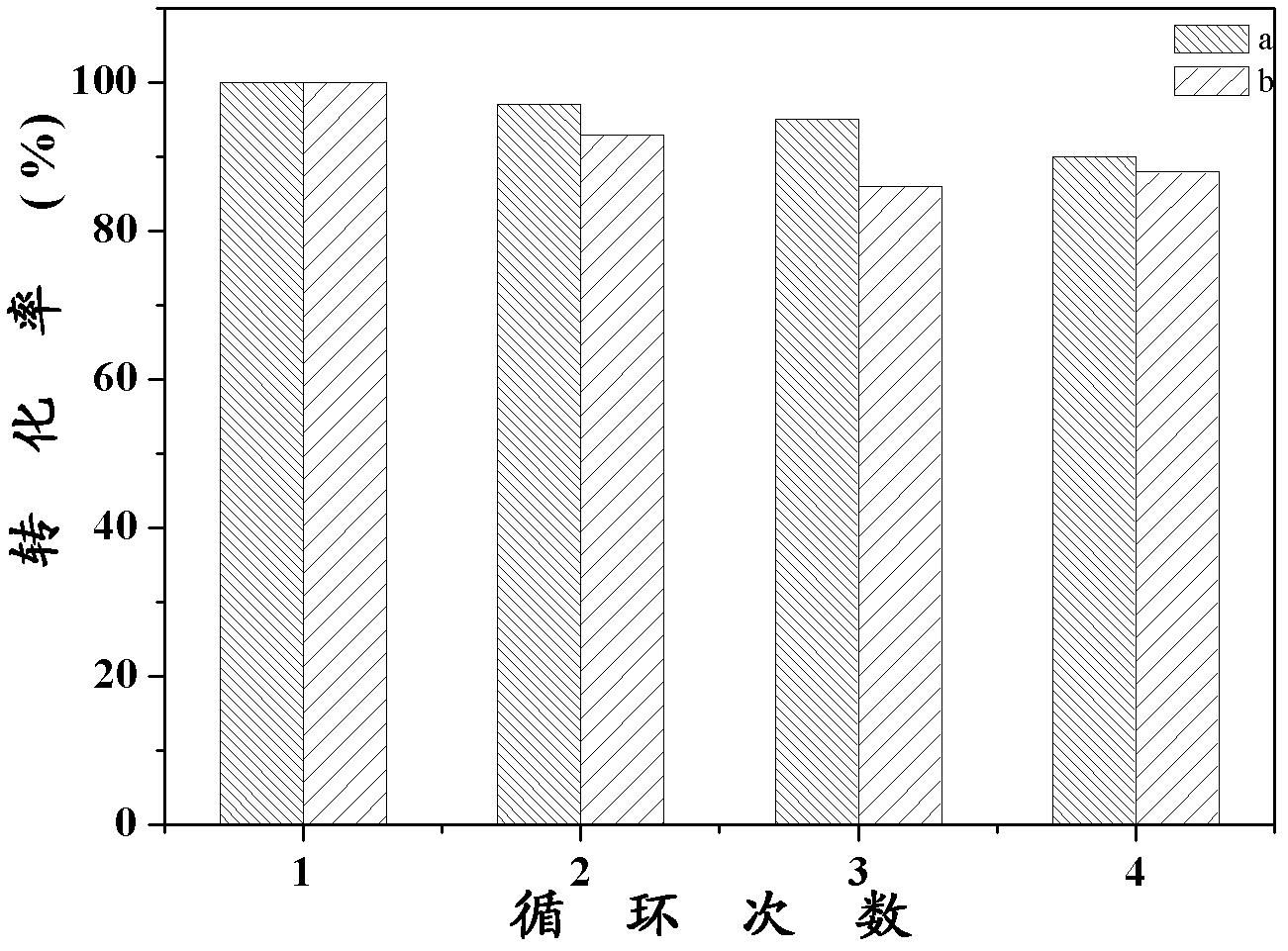
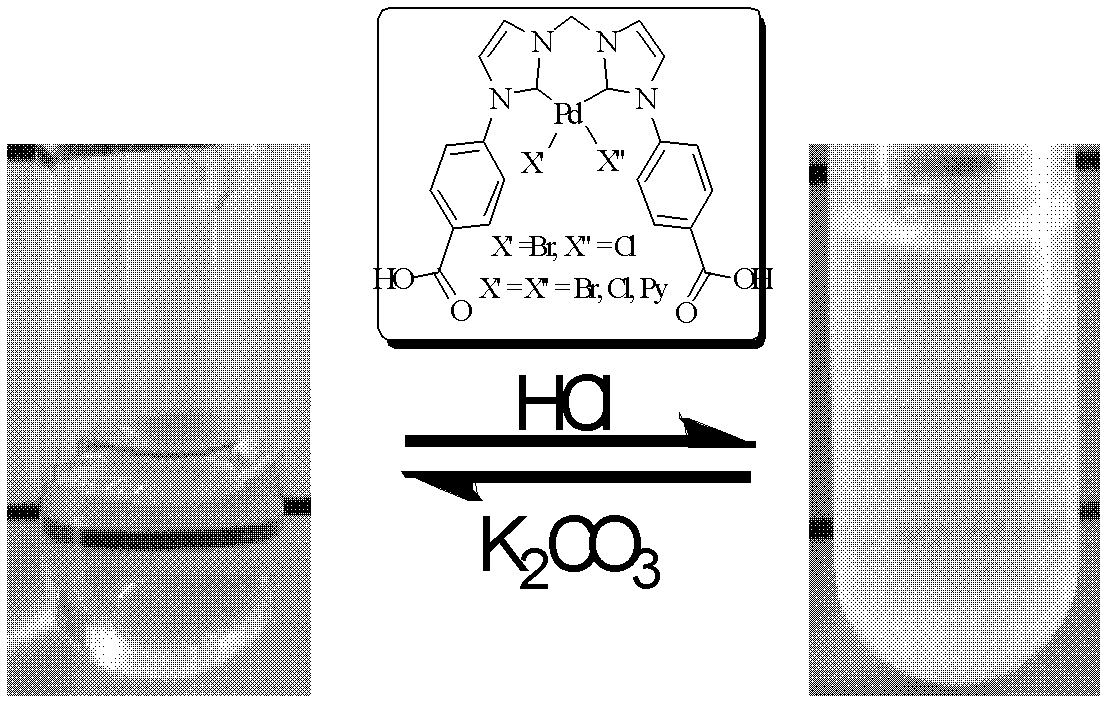
Synthesis of pH-responding palladium N-heterocyclic carbene chelate and catalytic application thereof
A heterocyclic carbene and chelate technology, which is applied in the synthesis and catalytic application of pH-responsive palladium N-heterocyclic carbene chelates, and can solve the problem of less research on catalytic reaction systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
[0018] At room temperature, under the protection of nitrogen, the ligand L1 (0.3g, 0.5mmol) and palladium acetate (0.1g, 0.5mmol) were added to 5.0mL dimethylsulfoxide. Stir, heat to 60 degrees centigrade and react for 12 hours, then heat up to 130 degrees centigrade and continue to react for 2 hours. After cooling down, it was concentrated to dryness in vacuum at 90 degrees Celsius, washed 3 times with tetrahydrofuran and dried in vacuum to obtain the N-heterocyclic carbene chelate of palladium with a yield of 86%. 1 H NMR (400 MHz, d 6-DMSO): δ 8.3-7.8 (m, 12H), 6.7-6.5 (m, 2H), 4.39 (q, J = 6.8 Hz,4H), 1.39 (t, J = 7.2 Hz, 4H); 13 C NMR (100 MHz, d 6-DMSO): δ 165.8, 159.9, 143.4, 130.5, 129.8, 125.9, 123.2, 61.5, 14.7.
Embodiment 2
[0020]
[0021] at room temperature, the 1 (0.3g, 0.4mmol) and lithium hydroxide (0.05g, 1.3 mmol) were added to a mixed solvent of 3 mL methanol and 3 mL water, stirred and reacted for 2 hours, concentrated to remove methanol, in an ice-water bath, dilute hydrogen bromide Acidify the acid until the pH value reaches 4, and a white solid is precipitated. The white solid is washed twice with 5 mL of water and 5 mL of tetrahydrofuran respectively, and dried in vacuum to obtain a white pH-responsive palladium N-heterocyclic carbene chelate 2 , the yield is 80 %; 1 H NMR (400 MHz, d 6-DMSO): δ 8.30-8.15 (m, 4H), 8.00-7.80 (m, 8H), 6.60-6.45 (m, 2H); 13 C NMR (100 MHz, d 6-DMSO): δ 167.3, 159.9, 143.1, 130.9, 130.6, 125.7, 123.1. Elemental analysis theoretical value: C 21 h 16 Br 2 N 4 o 4 Pd·H 2 O (672.6): C 37.50, H 2.70, N 8.33, measured by elemental analysis: C 37.48, H 2.89, N 8.21 %.
Embodiment 3
[0023]
[0024] at room temperature, the 1 (0.3g, 0.4mmol) and lithium hydroxide (0.05g, 1.3 mmol) were added to a mixed solvent of 3 mL methanol and 3 mL water, stirred and reacted for 2 hours, concentrated to remove methanol, acidified with dilute hydrochloric acid in an ice-water bath When the pH value reached 4, a white solid was precipitated, and the white solid was washed twice with 5 mL of water and 5 mL of tetrahydrofuran respectively, and dried in vacuum to obtain a white pH-responsive palladium N-heterocyclic carbene chelate 3 , yield 73 %; 1 H NMR (400 MHz, d 6-DMSO): δ 8.30-8.15 (m, 4H), 8.10-7.80 (m, 8H), 6.60-6.45 (m, 2H); 13 C NMR (100 MHz, d 6-DMSO): δ 167.3, 158.6, 143.0, 130.8, 130.6, 125.5, 125.5, 123.2, 123.0. Elemental analysis theoretical value: C 21 h 16 BrClN 4 o 4 Pd 0.5H 2 O (619.2): C 40.74, H 2.77, N 9.05, measured by elemental analysis: C 40.76, H 2.97, N 8.95 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com