Primer set for detection of Mycobacterium tuberculosis and its resistance to pyrazinamide
A Mycobacterium tuberculosis, pyrazinamide-resistant technology, which is applied in the biological field to achieve the effects of high sensitivity, shortened time, and short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
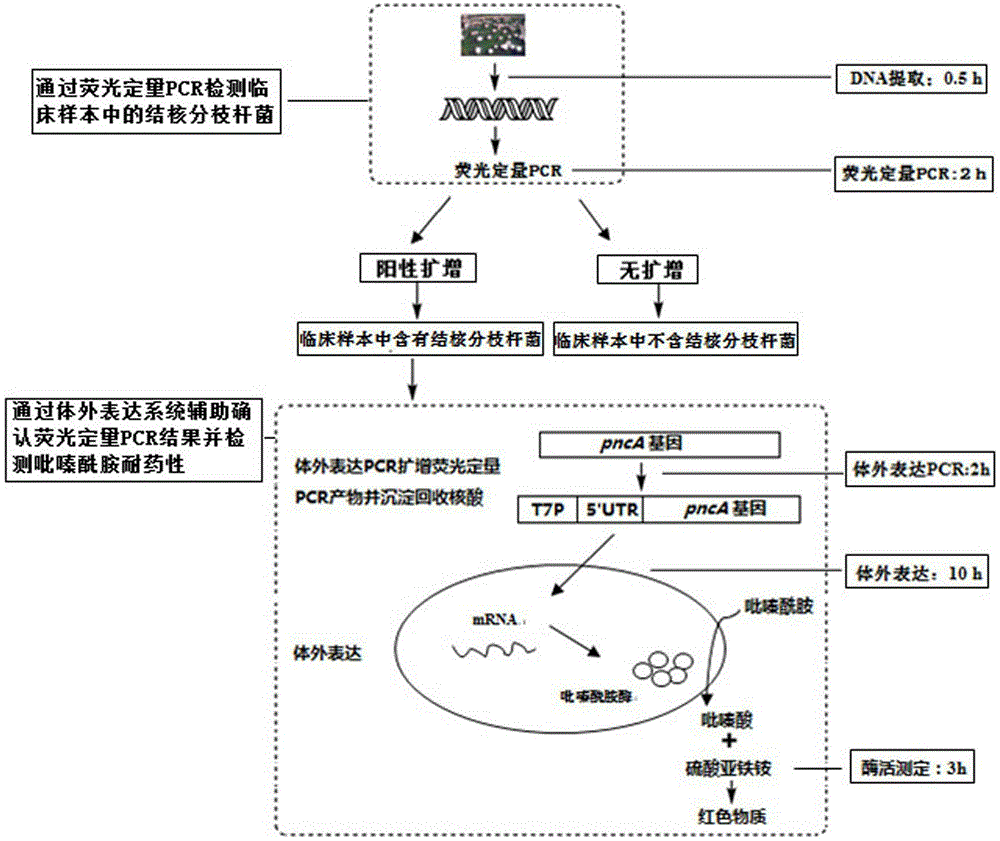
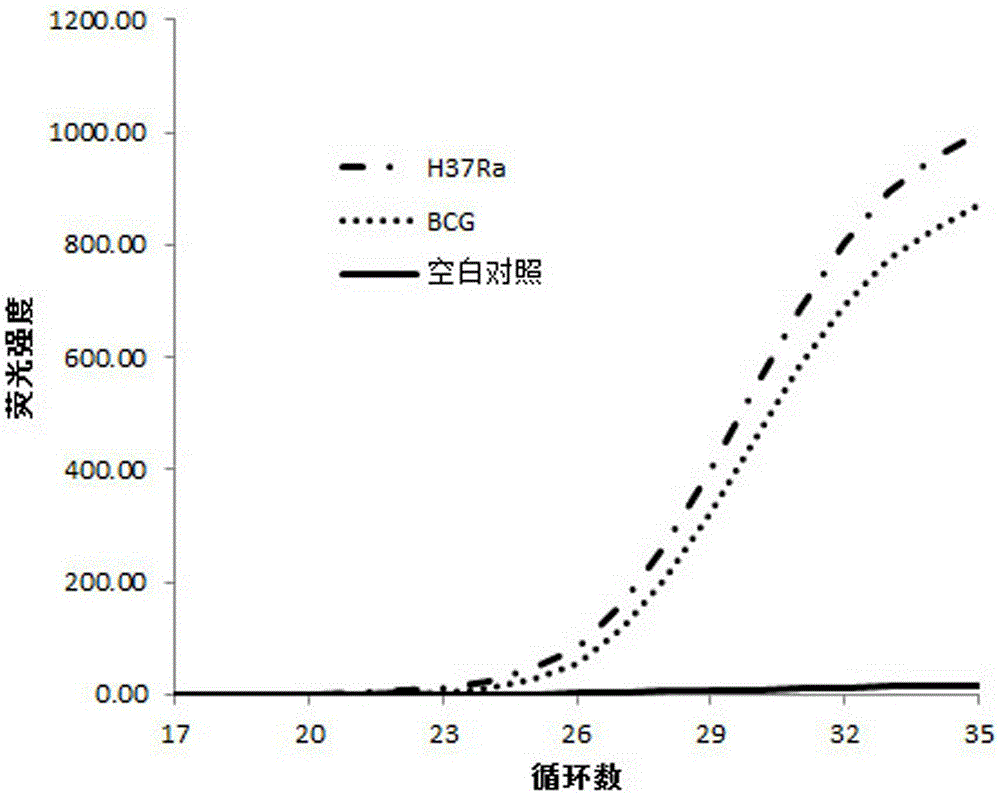
[0064] The pyrazinamide-sensitive strain Mycobacterium tuberculosis standard strain M.tuberculosisH37Ra (American Culture Collection Center No.: ATCC25177) was used as the pyrazinamide drug-sensitive control, and the standard pyrazinamide-resistant strain BCG (American Culture Collection Center No.: ATCC19274) as pyrazinamide drug resistance control, the assay results of these two cultured tuberculosis strains are used to establish a fluorescent quantitative PCR standard curve for judging whether the actual sample contains Mycobacterium tuberculosis and test whether this method can distinguish the standard pyrazinamide Zinamide-resistant and susceptible strains.
[0065] Specific steps are as follows:
[0066] 1. Lysis of Mycobacterium tuberculosis and extraction of its DNA:
[0067] (1) From the L-J medium cultured with the standard strains of Mycobacterium tuberculosis M.tuberculosisH37Ra and BCG, scrape a ring of bacteria with an inoculation loop, dissolve in 2mL NaOH soluti...
Embodiment 2
[0096] In this embodiment, 3 parts of clinical sputum (No. 218, 271, and No. 8333) of tuberculosis patients diagnosed as tuberculosis patients collected from Wuhan Tuberculosis Prevention and Control Institute are used as test samples to test whether this method can be suitable for the classification of tuberculosis in clinical sputum. The detection of mycobacteria and the detection of pyrazinamide resistance were initially verified. Specific steps are as follows:
[0097] 1. Cracking and extracting the DNA of Mycobacterium tuberculosis in sputum;
[0098] (1) Add 2mL NaOH solution to a certain amount of sputum (1-5mL), mix well, and incubate at 37°C for 20min.
[0099] (2) Centrifuge for 10 minutes, remove the supernatant and keep the precipitate. Wash with PBS, repeat the wash 2 times, remove the supernatant and save the precipitate for detection.
[0100] (3) DNA extraction: add purified water of DNA extraction solution to the above-mentioned processed specimen. Boiling...
Embodiment 3
[0121] In the step of pyrazinamide resistance detection, it is necessary to add in vitro expression elements, such as T7 promoter, expression enhancer, etc., to the fluorescent quantitative PCR product. In this embodiment, the pyrazinamide-sensitive strain Mycobacterium tuberculosis standard strain M.tuberculosisH37Ra (American Culture Collection Center No.: ATCC25177) is used as a pyrazinamide drug-sensitive control, and the standard pyrazinamide-resistant strain BCG (American Culture Collection Center) Center No.: ATCC19274) as pyrazinamide resistance control, two strains were used for in vitro expression PCR with and without primers of an expression enhancer 5'UTR. Then it was added to the wheat germ cell-free expression system to study whether the expression enhancer in the primer would affect the determination of pyrazinamide resistance.
[0122] Specific steps are as follows:
[0123] 1. Using the same method as in Example 1 to lyse Mycobacterium tuberculosis standard s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com