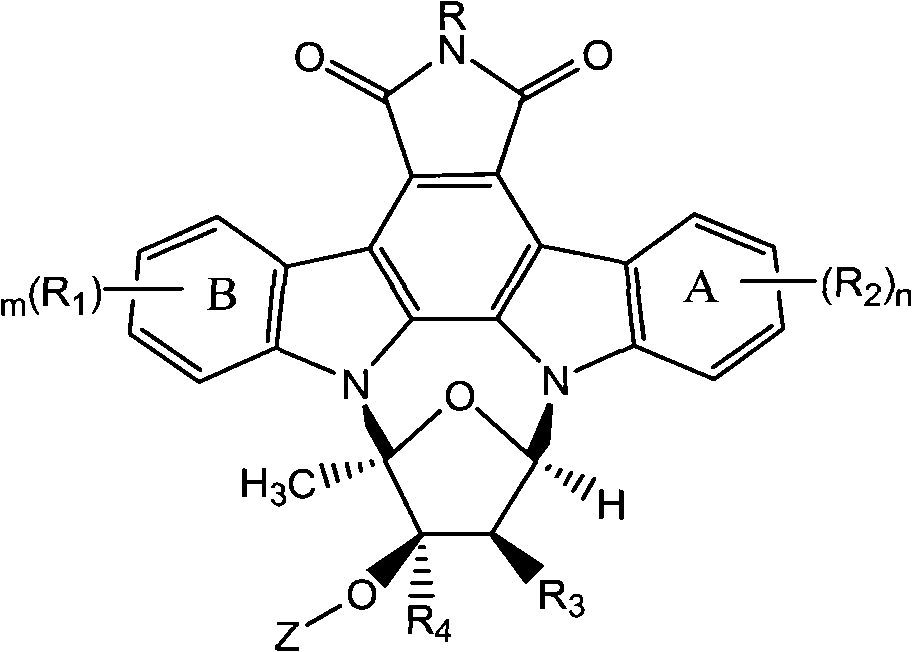
Semisynthetic method of staurosporine derivative
A technology of staurosporine and derivatives, applied in the field of semi-synthesis of staurosporine derivatives, to achieve the effect of cheap and easy-to-obtain raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
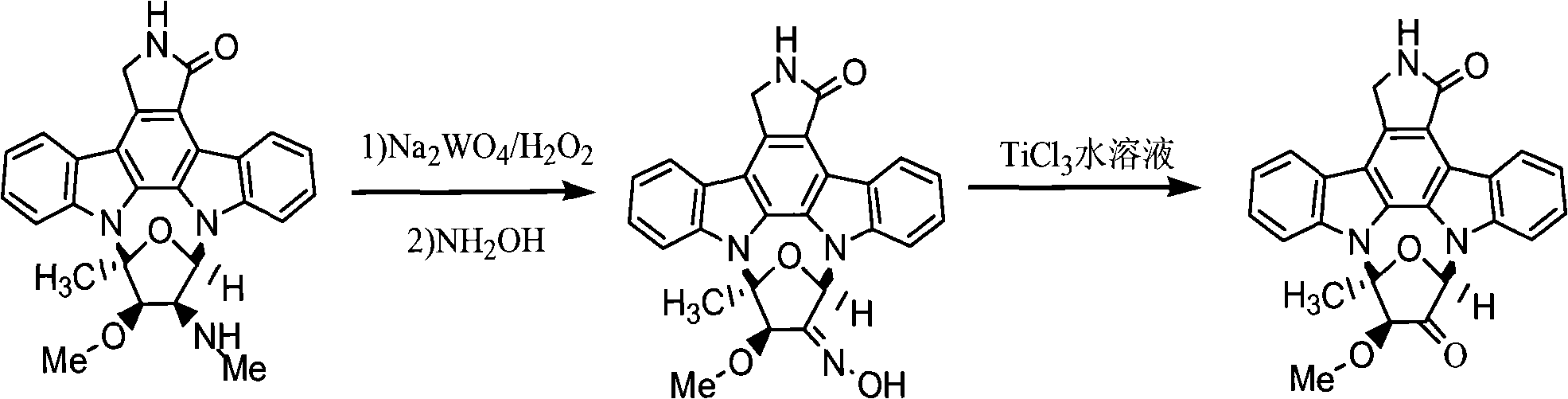
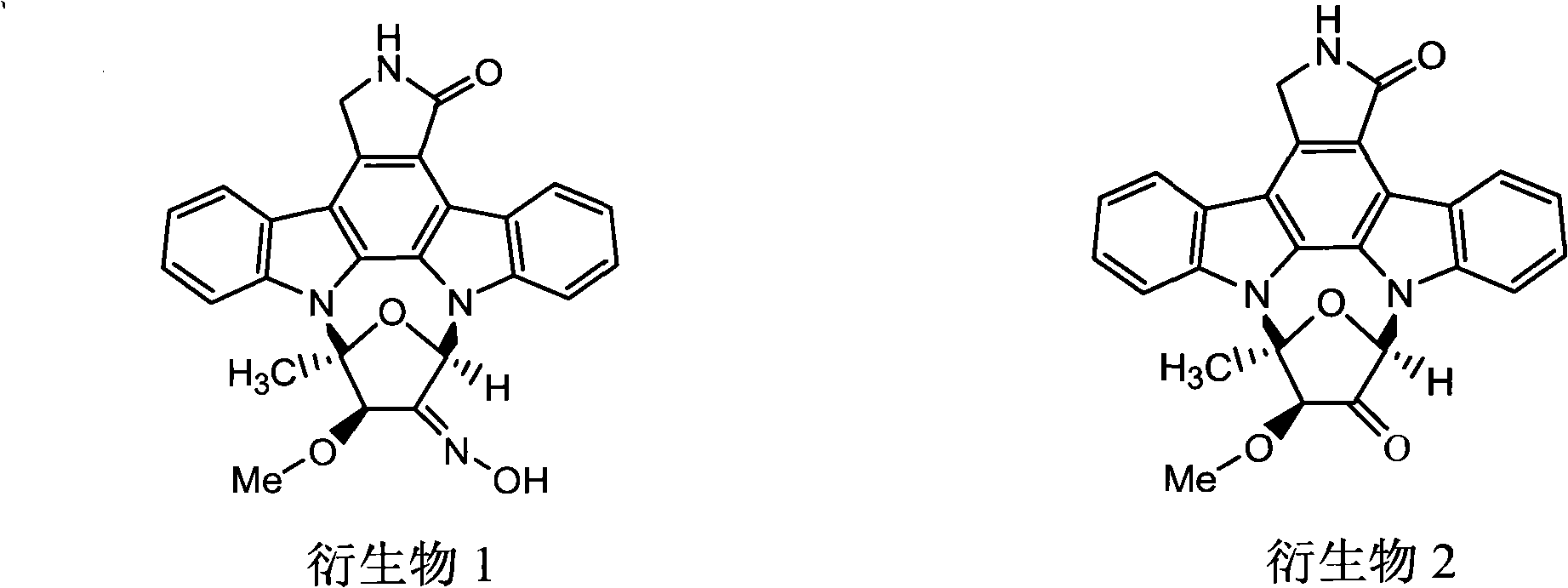
[0028] Take 5g of fermented staurosporine products as raw material, dissolve it in a mixture of 120ml of methanol and 120ml of dichloromethane, add 0.5g of sodium tungstate dihydrate and 4ml of 30% aqueous hydrogen peroxide solution, and keep it away from light at room temperature Stir for 20 h, cool to 0°C, add 150 ml of saturated aqueous sodium bisulfite solution to terminate the reaction, evaporate the solvent under reduced pressure to obtain a yellow oil. The substance was dissolved in a mixture of 80ml methanol and 80ml dichloromethane, stirred at 0°C, added 1.5g hydroxylamine hydrochloride and 12ml pyridine, diluted with water, added 120ml dichloromethane, extracted twice, the organic layer was washed with water, anhydrous sodium sulfate Drying, concentration under reduced pressure, methanol crystallization to obtain light yellow crystals, recrystallization in ethyl acetate-methanol (5:1) to obtain colorless crystals, staurosporine derivative 1, yield 75.3%.
[0029] Der...
Embodiment 2
[0031] Take 5g of fermented staurosporine products as raw materials, dissolve it in a mixture of 120ml ethanol and 120ml dichloromethane, add 0.5g sodium tungstate dihydrate and 4ml 30% hydrogen peroxide aqueous solution, and keep it away from light at room temperature Stir for 20 h, cool to 0°C, add 150 ml of saturated aqueous sodium bisulfite solution to terminate the reaction, evaporate the solvent under reduced pressure to obtain a yellow oil. The substance was dissolved in a mixture of 80ml ethanol and 80ml dichloromethane, stirred at 0°C, added 1.5g hydroxylamine hydrochloride and 12ml pyridine, diluted with water, added 120ml dichloromethane, extracted twice, the organic layer was washed with water, anhydrous sodium sulfate Drying, concentration under reduced pressure, methanol crystallization to obtain light yellow crystals, recrystallization in ethyl acetate-methanol (5:1) to obtain colorless crystals, staurosporine derivative 1, yield 76.6%.
[0032] Derivative 1 was...
Embodiment 3
[0034] Take 5g of fermented staurosporine as raw material, dissolve it in a mixture of 120ml of methanol and 120ml of chloroform, add 0.5g of sodium tungstate dihydrate and 4ml of 30% aqueous hydrogen peroxide solution, and keep it away from light at room temperature Stir for 20 h, cool to 0°C, add 150 ml of saturated aqueous sodium bisulfite solution to terminate the reaction, evaporate the solvent under reduced pressure to obtain a yellow oil. The substance was dissolved in a mixture of 80ml methanol and 80ml chloroform, stirred at 0°C, added 1.5g hydroxylamine hydrochloride and 12ml pyridine, diluted with water, added 120ml dichloromethane, extracted twice, the organic layer was washed with water, anhydrous sodium sulfate Drying, concentration under reduced pressure, methanol crystallization to obtain light yellow crystals, recrystallization in ethyl acetate-methanol (5:1) to obtain colorless crystals, staurosporine derivative 1, yield 76.8%.
[0035] Derivative 1 was disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com