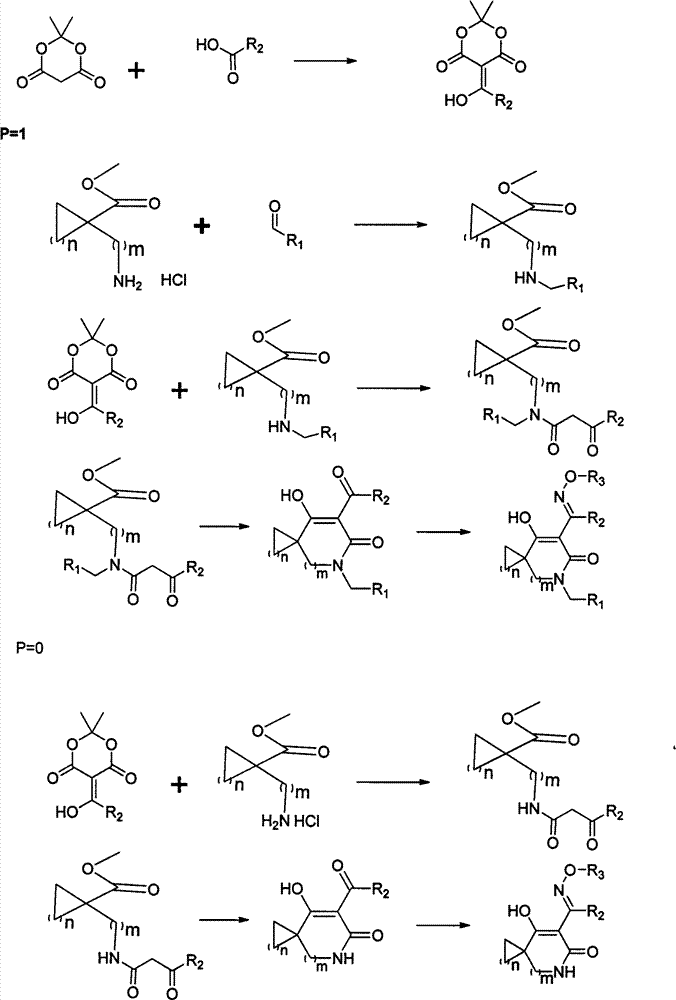
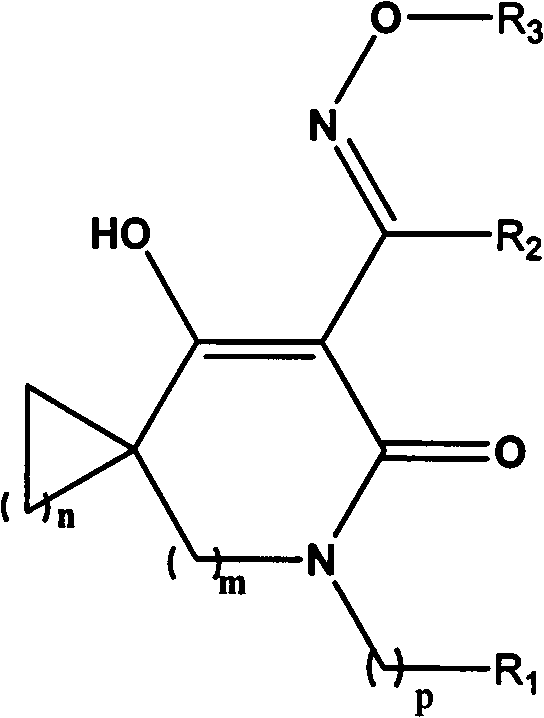
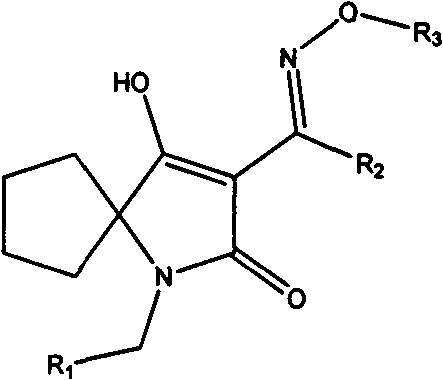
Oxime ether derivatives of spirocyclic tetronic acid, the preparation thereof, and insecticidal, acaricidal, bactericidal and herbicidal usages thereof
A technology of spirotetronate oxime and its derivatives, which can be used in botany equipment and methods, chemicals for biological control, pesticides, etc., and can solve the problems that have not been reported in relevant literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1 7-Hydroxy-6-(1-(methoxyimino)ethyl)-4-azaspiro[2.3]hexane-6-en-5-one
[0023] 1. Preparation of 5-acetyl Michaelis acid
[0024] In a 50mL round-bottomed flask, add 25mL of dichloromethane, then add 1mmol of acetic acid, add 1-1.5mmol of dehydrating agent DCC or EDCI, 0.1-1mmol of DMAP, and then add 1mmol of Michaelis acid, and control the reaction temperature at 0°C to room temperature. 8 to 17 hours, TLC follow-up monitoring, until the reaction stops. Then wash with hydrochloric acid, dry with anhydrous sodium sulfate or anhydrous magnesium sulfate, and directly make the raw material for the next step.
[0025] 2. Methyl 1-(3-carbonylbutyrylamino)cyclopropanecarboxylate
[0026] Add 25mL of ethyl acetate or acetonitrile into a 50mL round bottom flask, then add 1mmol of methyl 1-aminocyclopropanecarboxylate hydrochloride, stir for 5-10 minutes, add 1-1.1mmol of triethylamine, and then add 5- Acetyl Michaelis acid 1-1.5mmol, react at 50-100°C for 3-8 hours,...
example 2
[0031] Example 2 8-Hydroxy-7-(1-(methoxyimino)ethyl)-4-azaspiro[3.3]heptane-6-en-5-one
[0032] 1, 5-acetyl Michaelis acid preparation (referring to example 1.1)
[0033] 2. Methyl 1-(3-carbonylbutyrylamino)cyclobutylcarboxylate
[0034] Add 25mL of ethyl acetate or acetonitrile into a 50mL round bottom flask, then add 1mmol of methyl 1-aminocyclobutanecarboxylate hydrochloride, stir for 5-10 minutes, add 1-1.1mmol of triethylamine, and then add 5- Acyl Michaelis acid 1-1.5mmol, react at 50-100°C for 3-8 hours, and concentrate under reduced pressure. V (ethyl acetate): V (petroleum ether) = 1: 3 to 1: 0.5, and column chromatography was performed to obtain the product.
[0035] 3. 7-Acetyl-8-hydroxy-5-azaspiro[3.4]octane-7-en-6-one
[0036] Add methanol or ethanol to a 50mL round bottom flask, add 1mmol of methyl 1-(3-carbonylbutyrylamino)cyclobutyrate, add 1-1.5mmol of sodium methoxide or sodium ethoxide, and control the reaction temperature at 65-100°C. Concentrate under ...
example 3
[0039] Example 3 3-Hydroxy-2-(1-(methoxyimino)ethyl)-1-azaspiro[3.4]octane-3-en-2-one
[0040] 1, 5-acyl Michaelis acid preparation (referring to example 1.1)
[0041] 2. Methyl 1-(3-carbonylbutyrylamino)cyclopentylcarboxylate
[0042] Add 25mL of ethyl acetate or acetonitrile into a 50mL round bottom flask, then add 1mmol of 1-aminocyclopentylcarboxylic acid methyl ester hydrochloride, stir for 5-10 minutes, add 1~1.1mmol of triethylamine, and then add 5-acetyl 1 to 1.5 mmol of Metzler's acid, react at 50 to 100°C for 3 to 8 hours, and concentrate under reduced pressure. V (ethyl acetate): V (petroleum ether) = 1: 3 to 1: 0.5, and column chromatography was performed to obtain the product.
[0043] 3. 3-Acetyl-4-hydroxy-1-azaspiro[3.4]octane-3-en-2-one
[0044] Add methanol or ethanol to a 50mL round bottom flask, add 1mmol 1-(3-carbonylalkylamide) methyl cyclopentacarboxylate, add 1-1.5mmol of sodium methoxide or sodium ethoxide, and control the reaction temperature at 65-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com