Genetically engineering lactobacillus and application thereof
A technology of Lactobacillus and preservation number, applied in the field of mutant Lactobacillus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
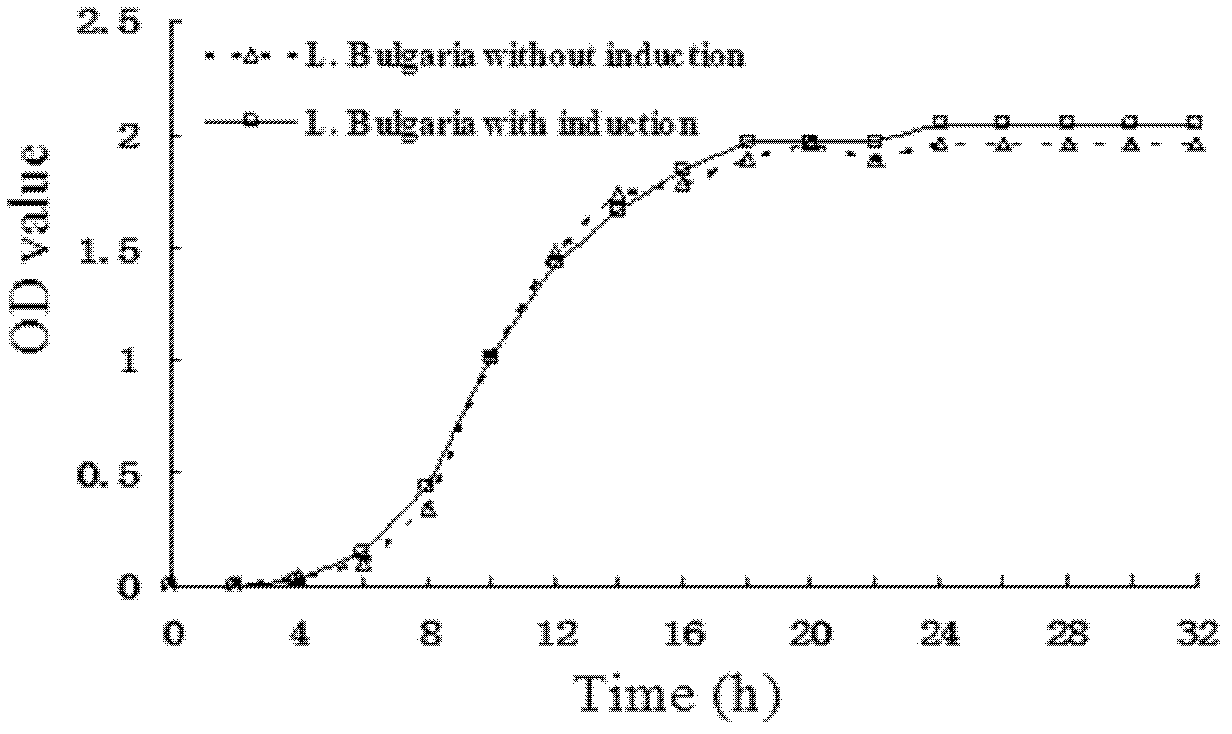
[0040] 1. Directional induction of Lactobacillus producing multiple uremic toxin-decomposing enzymes: inoculate activated L.B. in the serum of uremia patients at 4% (v / v), shake and mix well, and culture anaerobically for 24 hours to form the first generation. After mixing, take 1% and place it in the serum of a new uremia patient. After shaking and mixing, culture it anaerobically at 37° C. for 24 hours, which is the second generation. After repeated subculture to 25 generations, the bacteria were taken out to detect the ability of the bacteria to decompose creatinine and urea.
[0041] 2. Double and multiple mutagenesis of Lactobacillus producing multiple uremic toxin-decomposing enzymes
[0042] After the directional induction, the L.B inducer strain with the strongest ability to decompose urea toxin is selected as the starting strain, and double mutagenesis is carried out according to the mutagenesis scheme described in claim 4.
[0043] 1) Multiple UV mutagenesis: take t...
Embodiment 2
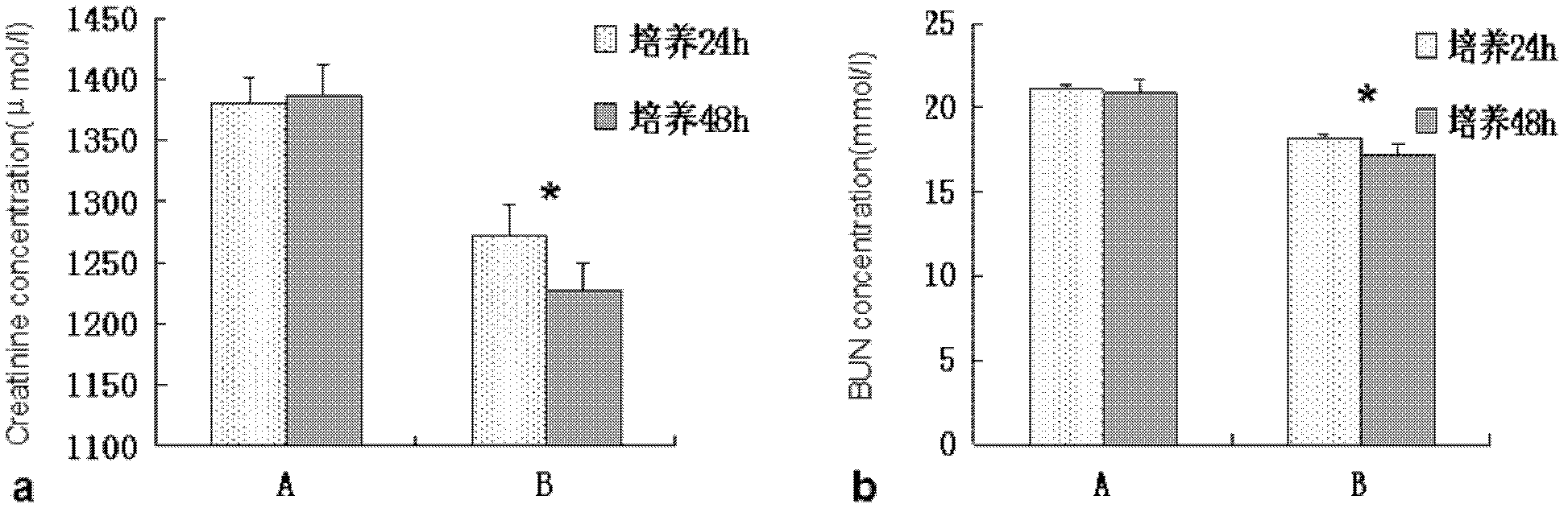
[0048] Determination of the ability of bacteria to decompose creatinine and urea: Activate the L.B obtained in Example 1 to make a bacterial solution, inoculate 4% in the MRS broth medium and mix well, then culture anaerobically at 37°C until the logarithmic phase Take it out, wash it repeatedly with sterile normal saline, centrifuge for 3 times, resuspend in normal saline, adjust the OD625 of the suspension in each tube to 0.08-0.1 by McFarland turbidimetry, about 1.5×108cfu / ml, take 200ul suspension bacteria each The solution was added to 800ul serum of uremic patients, cultured anaerobically at 37°C, taken out at 24h and 48h respectively, centrifuged at 40000rmp / min for 5min, and the supernatant was taken to detect the concentration of creatinine and urea nitrogen. Take 200ul of normal saline and add it to the above culture medium as a control group, and set 5 parallel tubes in each group. Jaffe's method and UV-GLDH method were used before and after cultivation, and the con...
Embodiment 3
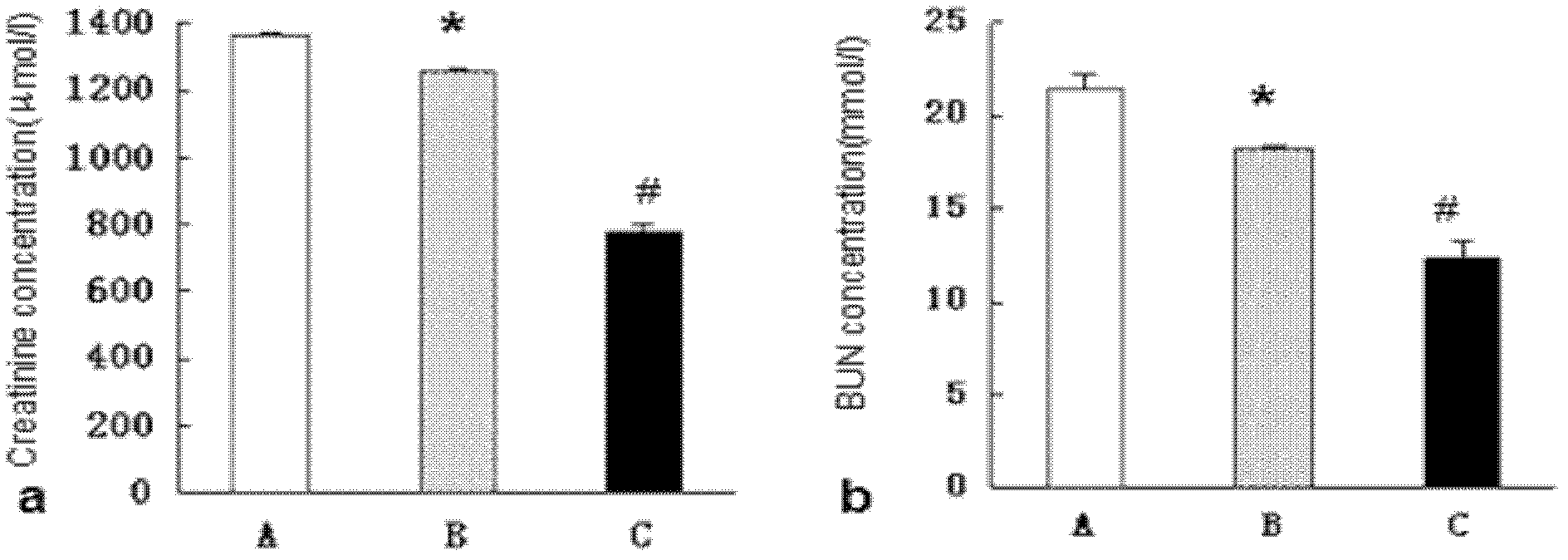
[0072] Observation on the curative effect of mutagenic strains in the treatment of experimental renal failure
[0073] Nephrectomy model establishment: 30 male 6-week-old SD rats, weighing 170-190g, were fed adaptively for 1 week, and the feed supply at the beginning of the experiment was determined according to the feed consumption during the week. Eight rats were randomly selected as the sham operation group, and the remaining 32 rats underwent 5 / 6 nephrectomy. Preparation method of 5 / 6 nephrectomy model: ① The first step is 2 / 3 left nephrectomy: after weighing, anesthetize with 0.35mL / 100g of 10% chloral hydrate, disinfect the skin in the middle of the lower abdomen, and draw a 1mL skin test needle Pierce the abdominal wall vertically, withdraw blood and urine, that is, inject. After successful anesthesia, the limbs were fixed on the operating board in prone position. First touch the left costal ridge angle, then make an upward and downward incision at the center of 1 cm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com