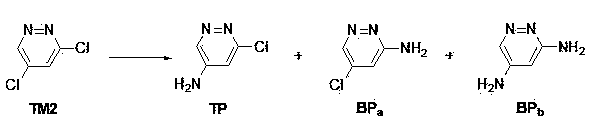
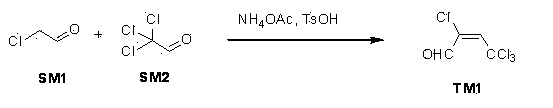Synthetic method of 3-chlorine-5-aminopyridazines serving as medicine and pesticide intermediates
A technology of aminopyridazine and synthesis method, which is applied in the field of synthesis of pharmaceutical and pesticide intermediates, can solve the problems of large waste acid waste gas emission, unsuitable for industrial production, cannot be used, etc., achieves improvement of total yield, avoids high carcinogenic chromium Metal, the effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Heat a mixture of 147.5 grams (1.00 moles) of chloral, 200 milliliters of toluene and 4.9 grams (0.064 moles) of ammonium acetate to reflux (the internal temperature is 87 ° C), and start to drop a 40% mass fraction of chloroacetaldehyde aqueous solution , and use a water separator to separate the water. The amount of addition is 127 grams (0.65 moles) of 100% chloroacetaldehyde. After the addition is complete, reflux and stir for 3 hours, add 9.8 grams of p-toluenesulfonic acid (0.057 moles), and continue to reflux for 4 hours , stop responding. The reaction solution was cooled to room temperature, evaporated to dryness, added 5000 ml of dichloromethane to dilute and filtered, the filtrate was concentrated under reduced pressure, the residue was distilled under reduced pressure, and the fraction at 90-100°C was collected under 12 mm Hg to obtain product 2. 4,4,4-Tetrachlorocrotonaldehyde is 78 grams of yellow oily liquid. The yield is 75%.
example 2
[0090] Dissolve 207.5 grams (1.00 moles) of 2,4,4,4-tetrachlorocrotonaldehyde in 1100 milliliters of tetrahydrofuran, cool to 15°C, add dropwise 118 grams (1.06 moles) of semicarbazide hydrochloride, 440 milliliters of water, and 150 milliliters of tetrahydrofuran The mixture was added dropwise under temperature control at 15°C. Stirring was maintained at this temperature for 5 hours. The reaction liquid was concentrated under reduced pressure, THF was removed, 3000 ml of water was added, the temperature was lowered to 5°C, a solid precipitated, filtered, and the filter cake was recrystallized with dichloromethane petroleum ether to obtain pure 3,5-dichloropyridazine as a white solid 90 gram. The yield is 60%. 1 HNMR (600MHz, CDCl 3 ) δ ppm 7.61 (s, 1H), 9.14 (s, 1H).
example 3
[0092] Dissolve 149 grams (1.00 moles) of 3,5-dichloropyridazine in 750 milliliters of 1,4-dioxane, add 680 milliliters of 25% concentrated ammonia (10 moles), and place in a stainless steel reactor , after sealing, the temperature was raised to 60°C, and the reaction was carried out for 8 hours. The reaction solution was cooled to room temperature and suction filtered, the filtrate was extracted with ethyl acetate (1000 ml × 3), the organic phase was dried and concentrated to obtain a gray solid crude product, which was recrystallized from ethanol to obtain an off-white solid 3-chloro-5-aminopyridazine 104.5 gram. The yield is 81%. 1 HNMR (600MHz, d 6 -DMSO) δ ppm 6.65 (d, 1H, J =2.4), 6.83 (s, 2H), 8.48 (d, 1H, J =2.4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com