Compound having alpha-glucosidase inhibitory activity in lotus leaves and application
A technology for glucosidase and inhibitory activity, applied in the field of preparation of drugs for the treatment of diabetes, can solve problems such as difficulty in finding α-glucosidase inhibitors, achieve the goal of reducing blindness, shortening screening time, and improving screening efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
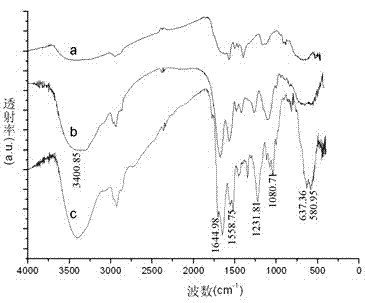
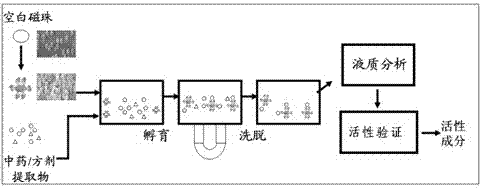
[0025] Example 1 Preparation of magnetic beads bound to α-glucosidase
[0026] 1. Reagent and solution preparation:
[0027] α-glucosidase (Saccharomyces cerevisiae, simga company); carboxyl magnetic beads Dynabeads® MyOne Carboxylic Acid, specification: 10 mg / ml (invitrogen company); 1-ethyl-3-(3-dimethylaminopropyl) carbon Diimine hydrochloride (EDC, sigma company); 2-(N-morpholine) ethanesulfonic acid (MES, sigma company); N-hydroxysuccinimide (NHS, sigma company).
[0028] Preparation of 25 mmol / L MES solution Weigh an appropriate amount of MES, add water to dissolve, add 1 mol / L sodium hydroxide solution to adjust the pH to 6, and prepare a MES solution with a concentration of 25 mmol / L.
[0029] Preparation of 50 mg / ml EDC solution Weigh an appropriate amount of EDC and quickly dissolve it in cold 25mmol / L MES solution to prepare an EDC solution with a concentration of 50mg / ml. Prepared fresh just before use.
[0030] Preparation of 50 mg / ml NHS solution Weigh an appr...
Embodiment 2
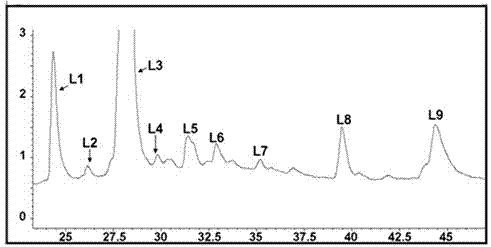
[0040] Example 2 Screening of Compounds with α-Glucosidase Inhibitory Activity in Lotus Leaf Extract
[0041] 1. Preparation of drugs, reagents and solutions:
[0042] Rutin standard (batch number: 100080-200707), National Institute for the Control of Pharmaceutical and Biological Products; Narciscoside standard (batch number: 111209), quercetin standard (batch number: 090426) Shanghai Ronghe Pharmaceutical Technology Development Co., Ltd.; Kaempferol -3-O-β-D-glucuronide standard substance (batch number: P0141), Shanghai Chunyou Biotechnology Co., Ltd.; p-nitrophenyl-α-D-galactopyranoside, sigma company.
[0043] Lotus leaf extract: take lotus leaf and add 70% ethanol to reflux extraction twice, combine the extracts, recover ethanol under reduced pressure, load the concentrated solution on D101 macroporous resin, elute with water, 60% ethanol, and 95% ethanol respectively, discard Remove the eluent from water, combine the 60% ethanol and 95% ethanol eluent, recover the solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com