Omeprazole sodium crystal compound and medicine composition containing omeprazole sodium crystal compound
A technology of omeprazole sodium and crystal compound, which is applied in the field of omeprazole sodium crystal compound and pharmaceutical composition containing the crystal compound, can solve the problems of allergic reaction, edema, phlebitis, tissue hypoxia and the like, and achieves The effect of high product purity, good dispersibility and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] [Example 1] Preparation of Omeprazole Sodium Crystalline Compound
[0061] 1) Dissolve 2.1kg of omeprazole sodium in 10L of water to obtain a 0.21g / mL aqueous solution of omeprazole sodium, filter it, and use the filtrate for later use;
[0062] 2) At room temperature, add the filtrate obtained in step 1) into a mixed solution of 205L acetone and isopropanol with stirring, wherein the volume ratio of acetone and isopropanol is 1:3.5, and cool down to 3°C, after obtaining crystals, continue to stir for 10 min; filter, wash the filter cake with water, and spray dry to obtain the crystalline compound of omeprazole sodium.
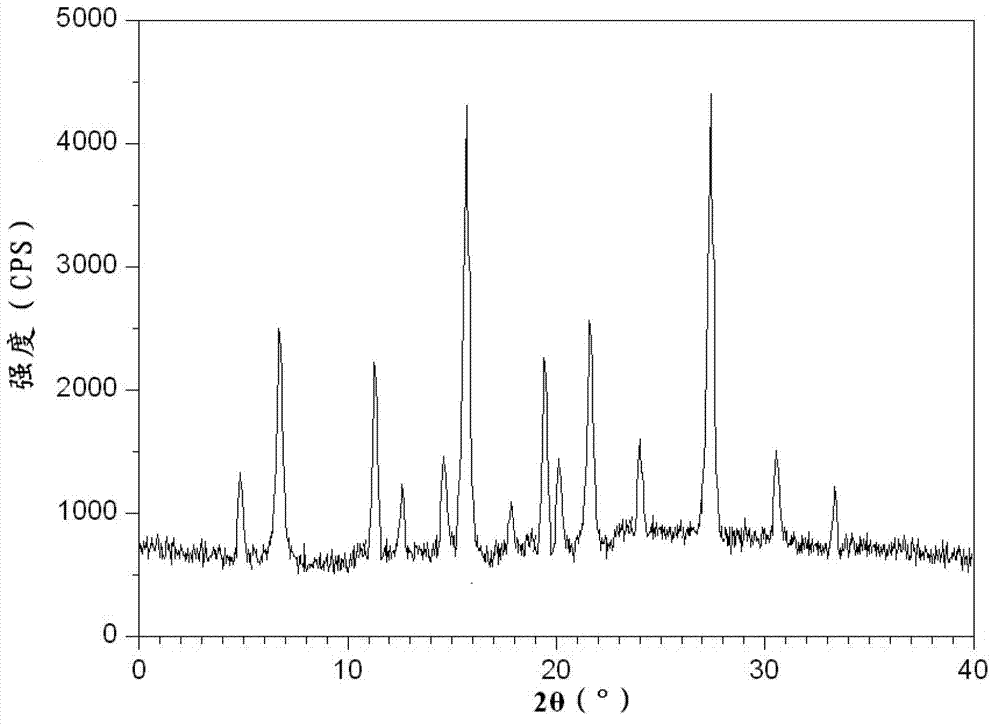
[0063] The obtained omeprazole sodium crystalline compound is measured by powder X-ray diffractometry, and the X-ray powder diffraction spectrum (see figure 1 ) showed characteristic diffraction peaks at 4.8°, 6.8°, 11.5°, 12.6°, 14.6°, 15.8°, 17.9°, 19.4°, 20.2°, 21.8°, 24.0°, 27.4°, 30.5° and 33.3°.
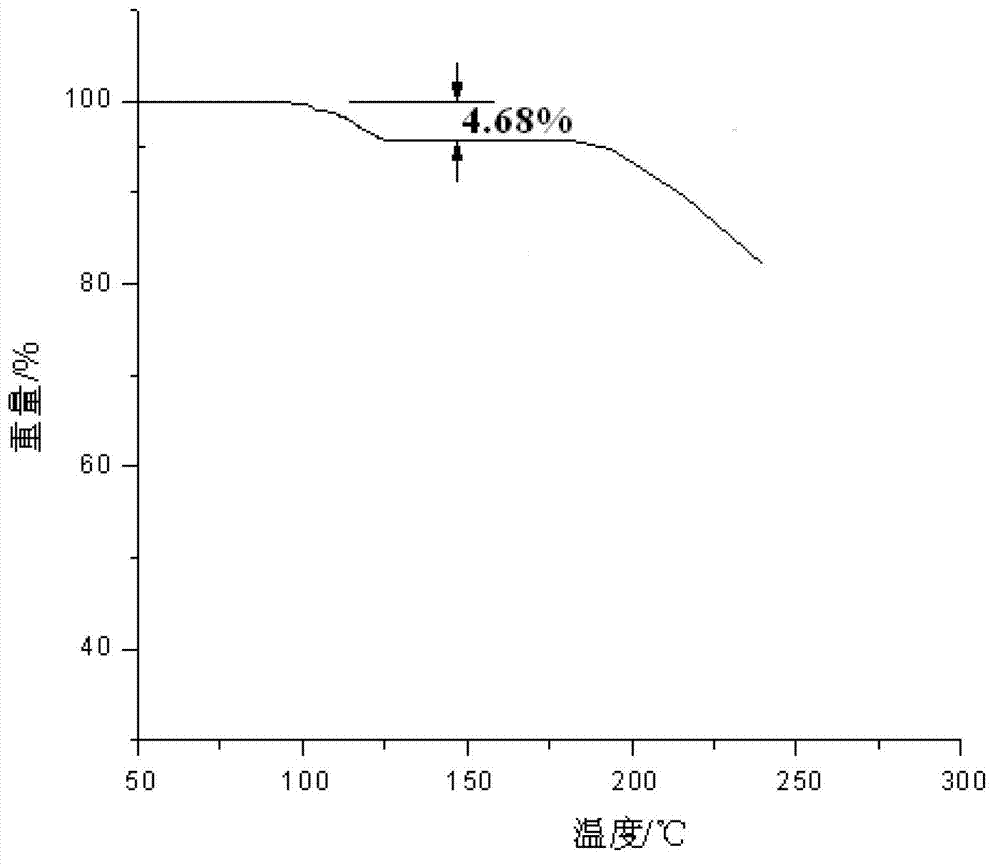
[0064] Adopt the PE Pyris Diamond TG thermal ana...
Embodiment 2-8
[0067]
[0068] The obtained omeprazole sodium crystalline compound of embodiment 2-8 is measured with powder X-ray diffractometry, and the X-ray powder diffraction pattern that expresses with 2θ ± 0.2 ° diffraction angle and adopts U.S. Perkin-Elmer company PE Pyris Diamond The thermogravimetric analysis figure that TG thermoanalyzer obtains is all similar to embodiment 1.
preparation Embodiment 1
[0069] [Preparation Example 1] Preparation of Omeprazole Sodium Freeze-dried Powder Injection
[0070] Prescription: specification (20mg / bottle)
[0071]
[0072] Preparation Process:
[0073] 1) Add the omeprazole sodium crystalline compound prepared in Example 1 and mannitol in the prescription amount into the liquid mixing tank, add 800ml of water for injection and stir until completely dissolved, and adjust the pH to 10 with 0.15mol / L sodium citrate solution .0~11.0, add water for injection to 1000ml;
[0074] 2) Add activated carbon for needles with 0.05% of the total amount of the solution, stir for 15 minutes, filter and decarbonize;
[0075] 3) Fine filter the medicinal solution with a 0.22 μm sterile microporous membrane, and measure the pH value, content, and half-stopper;
[0076] 4) Freeze-drying: pre-freeze at -40°C for 2 hours, -40-10°C, dry under reduced pressure and vacuum for 25 hours, and dry at high temperature at about 35°C for 7 hours;
[0077] 5) A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com