Synthesis method of esomeprazole sodium
A technology for esomeprazole sodium and omeprazole sodium is applied in the field of esomeprazole sodium synthesis, and can solve the problems of many impurities, high synthesis cost, low esomeprazole content, and the like, To achieve the effect of simple synthesis process, low cost and lower synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
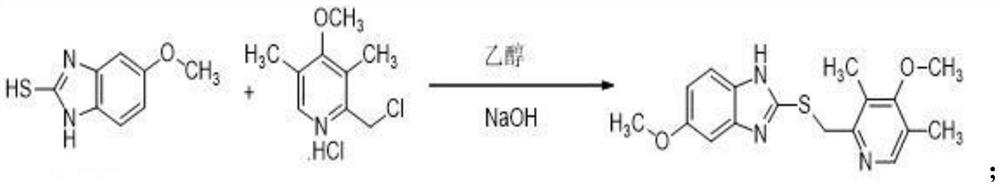
[0031] A kind of synthetic method of esomeprazole sodium, synthetic steps are as follows:
[0032] (1) preparation of omeprazole sulfide
[0033] 12kg of 5-methoxy-2-mercaptobenzimidazole was stirred in a mixed solution of ethanol (density 0.81g / ml, addition 46.8kg), sodium hydroxide solution (5.33kg sodium hydroxide, 14.4kg purified water) until Dissolve, add 14.79kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride after dissolving 5-methoxy-2-mercaptobenzimidazole, heat, stir and reflux until the end of the reaction, The reaction solution was obtained, wherein during the process of feeding and stirring, the temperature was controlled at 35° C., the reflux reaction time was 0.5 h, and after the reaction was completed, it was purified by crystallization of the organic phase.
[0034] Wherein, the organic phase is crystallized to be purified by adding the reaction liquid to the organic phase of ethyl acetate, adjusting the pH to 10 through sodium hydroxide soluti...
Embodiment 2
[0048] A kind of synthetic method of esomeprazole sodium, synthetic steps are as follows:
[0049] (1) preparation of omeprazole sulfide
[0050] 12kg of 5-methoxy-2-mercaptobenzimidazole was stirred in a mixed solution of ethanol (density 0.81g / ml, addition 46.8kg), sodium hydroxide solution (5.33kg sodium hydroxide, 14.4kg purified water) until Dissolve, add 14.79kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride after dissolving 5-methoxy-2-mercaptobenzimidazole, heat, stir and reflux until the end of the reaction, The reaction solution was obtained, wherein during the process of feeding and stirring, the temperature was controlled at 35° C., the reflux reaction time was 1.5 h, and after the reaction was completed, it was purified by crystallization of the organic phase.
[0051] Wherein, the organic phase is crystallized to be purified by adding the reaction solution to the organic phase of ethyl acetate, adjusting the pH to 11 through sodium hydroxide solu...
Embodiment 3
[0065] A kind of synthetic method of esomeprazole sodium, synthetic steps are as follows:
[0066] (1) preparation of omeprazole sulfide
[0067] 12kg of 5-methoxy-2-mercaptobenzimidazole was stirred in a mixed solution of ethanol (density 0.81g / ml, addition 46.8kg), sodium hydroxide solution (5.33kg sodium hydroxide, 14.4kg purified water) until Dissolve, add 14.79kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride after dissolving 5-methoxy-2-mercaptobenzimidazole, heat, stir and reflux until the end of the reaction, A reaction liquid was obtained, wherein during the process of feeding and stirring, the temperature was controlled at 35° C., the reaction time of reflux was 1 h, and after the reaction was completed, it was purified by crystallization of the organic phase.
[0068] Wherein, the organic phase is crystallized to be purified by adding the reaction liquid to the organic phase of ethyl acetate, adjusting the pH to 10 through sodium hydroxide solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com