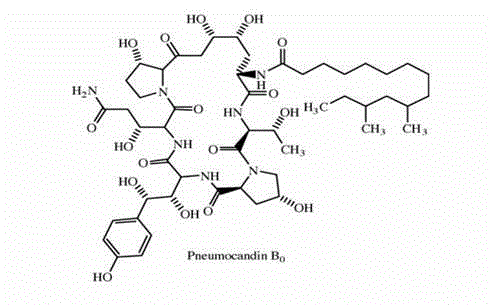
Method for purifying caspofungin precursor pneumocandin B0 component
A purification method and caspofungin technology, applied in the field of purification and caspofungin synthesis, can solve problems such as complicated operation, lower product yield, increased difficulty, etc., and achieve simple purification steps, high production feasibility, and equipment less demanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 5g PB 0 The crude components were dissolved in 500ml of 45% ethanol (V / V), which also contained 1‰ formic acid (V / V), and then the pH value was adjusted to 3.0 with formic acid, and 500ml of filtrate was obtained by filtration, and the filtrate was reversed HPLC analysis, the main peak (including PB 0 , PC 0 Components, the two show the same chromatographic peak in reversed-phase chromatography) The content of the component is 3.62g, normal phase HPLC analysis (PB 0 , PC 0 Components can be separated), PB 0 The content of the components was 2.43 g, and the above filtrate was applied to a reverse phase column.
[0029] C18 reversed-phase column material (Fuji Corporation, Japan) Specifications: Lot.No.HU00200, Pro. No.SMB100, Size: 20 / 45um.
[0030] Weigh 300g of C18 reversed-phase column material, load the column with 95% ethanol (V / V) solution, and then equilibrate the column with 45% ethanol, containing 1‰ formic acid ((V / V) solution. After loading, use 50% ethan...
Embodiment 2
[0035] 5g PB 0 The crude component was dissolved in 500ml of 45% ethanol (V / V) solution, then adjusted to pH 4.0 with formic acid, and filtered to obtain 500ml of filtrate, which was analyzed by reverse phase HPLC, the main peak (containing PB 0 , PC 0 Components, the two show the same chromatographic peak in reversed-phase chromatography) The content of the component is 3.62g, normal phase HPLC analysis (PB 0 , PC 0 Components can be separated), PB 0 The content of the components was 2.43 g, and the above filtrate was applied to a reverse phase column.
[0036] C18 reversed-phase column material (Fuji Corporation, Japan) Specifications: Lot.No.HU00200, Pro. No.SMB100, Size: 20 / 45um.
[0037]Weigh 300g of C18 reverse-phase column material, pack the column with 95% ethanol solution, and then equilibrate the column with 45% ethanol containing 1‰ formic acid ((V / V) solution. After loading the sample, use 50% ethanol (V / V) (Which contains 1‰ formic acid (V / V)) to wash the col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com