Quinacridone borate, and preparation method and application thereof
A technology of boronization and reaction, which is applied in the fields of chemical instruments and methods, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc. It can solve the problems of difficult stannization or boronization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
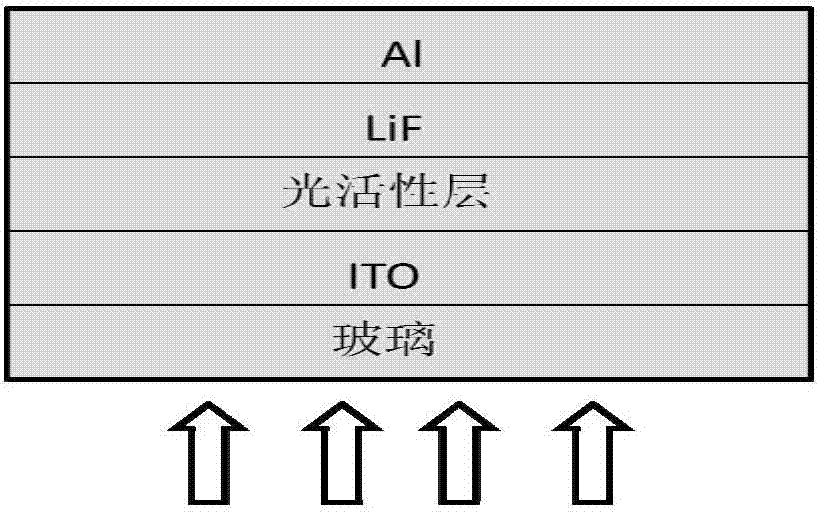
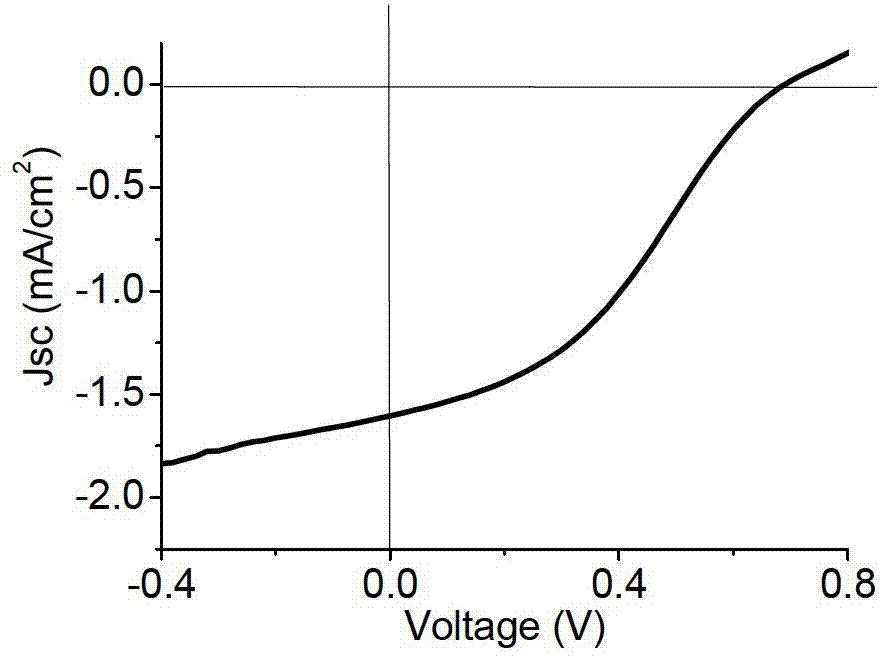
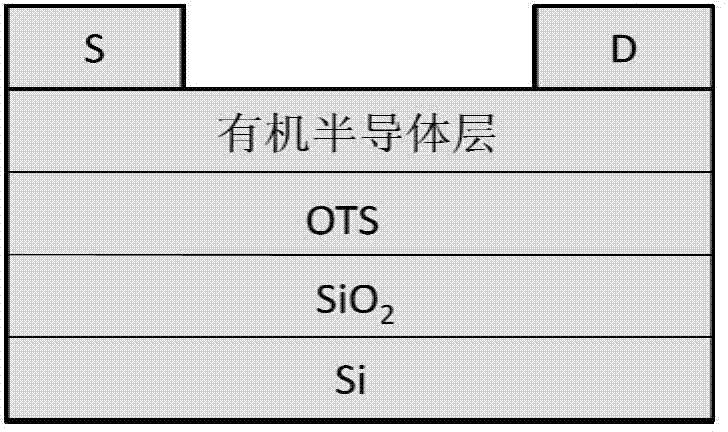
Image
Examples
Embodiment 1
[0080] Embodiment 1: the synthesis of quinacridone borate ester shown in formula I (R 1 for 2-octyl-dodecyl)
[0081] Synthesize compounds A-1 and B-1 first, and the structural formula is as follows:
[0082]
[0083] First, add 3 grams of quinacridone, 4.3 grams of tetrabutylammonium bromide, and 2.2 grams of sodium hydride into a 500 ml two-necked bottle, use toluene as a solvent, protect the temperature with argon, raise the temperature to 120 ° C for 2 hours, and drop 1 -Bromo-2-octyl-dodecane, cooled after reacting overnight, spin-dried toluene, and the solid was chromatographed on a silica gel column, eluted with petroleum ether: dichloromethane = 3: 1 to obtain a yellow-green powder that is A- 1. The yield is 35%. Molecular ion peak: 873.2.
[0084] In a 250 ml two-necked bottle, add 700 mg of A-1, 170 mg of potassium acetate, and 150 ml of acetic acid, heat to 80°C, and inject 0.1 ml of Br 2 After reacting for 4 hours, it was extracted with dichloromethane, chro...
Embodiment 2
[0090] Embodiment 2: the synthesis of quinacridone borate ester shown in formula I (R 1 for decanyl)
[0091] Synthesize compounds A-2 and B-2 first, and the structural formula is as follows:
[0092]
[0093] First, add 1 gram of quinacridone, 2.1 grams of tetrabutylammonium bromide, and 0.8 grams of sodium hydride into a 500 ml two-necked bottle, use toluene as a solvent, protect the temperature with argon, raise the temperature to 120 ° C for 2 hours, and drop 1 -Decane bromide, cooled after reacting overnight, spin-dried toluene, and the solid was subjected to silica gel column chromatography, eluting with petroleum ether: dichloromethane = 2:1 to obtain light green powder A-2 with a yield of 30%. Molecular ion peak: 592.6.
[0094] In a 250 ml two-necked bottle, add 400 mg of A-2, 0.8 mg of potassium acetate, and 100 ml of acetic acid, heat to 80°C, and inject 0.05 ml of Br 2 After reacting for 4 hours, it was extracted with dichloromethane, chromatographed on silic...
Embodiment 3
[0099] Embodiment 3: compound shown in preparation formula II (R 1 is 2-octyl-dodecyl, Ar is preferably Ar-1)
[0100]
[0101] The preparation of compounds D, E, and F is involved in the process of preparing Ar-1, and the structural formula is as follows:
[0102]
[0103] Preparation of compounds D, E, F:
[0104] Under strong basic conditions, cyanothiophene and dimethyl succinate react to obtain compound D;
[0105] Compound D reacts with 1-bromo-2-octyl-dodecane in DMF solution containing weak base and phase transfer catalyst to obtain E. In chloroform, compound E and N-bromosuccinimide are added to obtain F;
[0106] Specific preparation process reference: Macromolecules, 2010, 43, 821.
[0107] The reaction equation for the preparation of small molecules with quinacridone borates is shown below:
[0108]
[0109] The specific preparation process is: under the protection of argon, in a round bottom flask, add 54 mg of quinacridone borate, 100 mg of compound...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com