Preparation method of high-efficiency low-platinum catalyst for direct methanol fuel cell
A methanol fuel cell, a direct technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of reducing catalyst stability and catalytic activity, and achieve low platinum loading and methanol catalysis highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
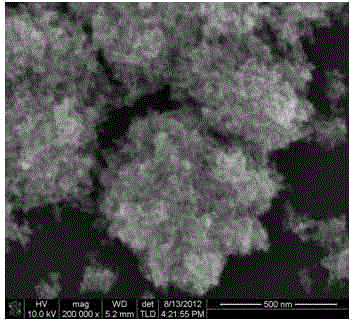
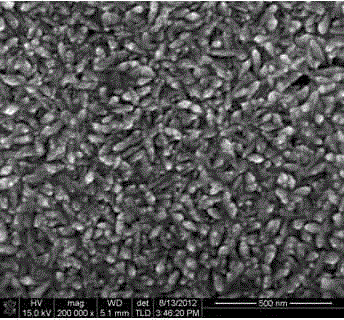
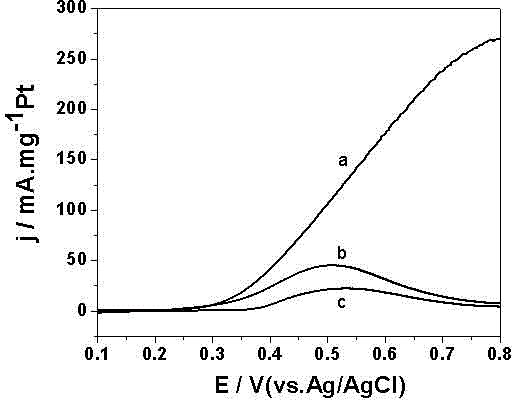
Image
Examples
Embodiment 1
[0034] (1), Titanium substrate pretreatment
[0035] The titanium sheet was first mechanically polished, then ultrasonically cleaned with deionized water, acetone, absolute ethanol and deionized water for 10 minutes, and finally dried at 60°C for 30 minutes for use;
[0036] (2) Preparation of Ni-P / Ti precursor electrode by pulse electrodeposition
[0037] A two-electrode system was adopted, the titanium substrate prepared in step (1) was used as the cathode, the platinum wire electrode was used as the anode, the electrolyte was an aqueous solution containing 50 g / L nickel sulfate and 50 g / L sodium hydrogenphosphite, and the electrodeposition temperature was 10 ℃, the pulse peak current density is 30A / dm 2 , the pulse on time is 1ms, the pulse off time is 10ms, and the electrodeposition time is 100s;
[0038] (3) Formation of Pt-Ni-P / Ti electrode by replacement method
[0039] Clean the Ni-P / Ti electrode prepared in step (2) with ultrapure water, then immerse in nitrogen-prot...
Embodiment 2
[0042] Step (1) is the same as step (1) in Example 1;
[0043] (2) Preparation of Ni-P / Ti precursor electrode by pulse electrodeposition
[0044] A two-electrode system was adopted, the titanium substrate prepared in step (1) was used as the cathode, the platinum wire electrode was used as the anode, the electrolyte was an aqueous solution containing 10 g / L nickel nitrate and 100 g / L potassium hydrogen phosphite, and the electrodeposition temperature was 70 ℃, the pulse peak current density is 1A / dm 2 , the pulse on time is 10ms, the pulse off time is 50ms, and the electrodeposition time is 5s;
[0045] (3) Formation of Pt-Ni-P / Ti electrode by replacement method
[0046] After cleaning the Ni-P / Ti electrode prepared in step (2) with ultrapure water, immerse it in the chloroplatinic acid aqueous solution under nitrogen protection for replacement, adjust the pH value of the chloroplatinic acid aqueous solution to 1, and the concentration to 0.1 g / L, Pt-Ni-P / Ti electrode was o...
Embodiment 3
[0050] Step (1) is the same as step (1) in Example 1;
[0051] (2) Preparation of Ni-P / Ti precursor electrode by pulse electrodeposition
[0052] A two-electrode system was adopted, the titanium substrate prepared in step (1) was used as the cathode, the platinum wire electrode was used as the anode, and the electrolyte was an aqueous solution containing 100 g / L nickel chloride and 10 g / L potassium hydrogen phosphite, and the electrodeposition temperature was 40℃, the pulse peak current density is 20A / dm 2 , the pulse on time is 0.1ms, the pulse off time is 1ms, and the electrodeposition time is 300s;
[0053] (3) Formation of Pt-Ni-P / Ti electrode by replacement method
[0054] After cleaning the Ni-P / Ti electrode prepared in step (2) with ultrapure water, immerse it in the chloroplatinic acid aqueous solution under nitrogen protection for replacement, adjust the pH value of the chloroplatinic acid aqueous solution to 7, and the concentration to 30 g / L, Pt-Ni-P / Ti electrode...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com