Iron-based amorphous alloy material with high glass-forming capability
A technology for iron-based amorphous and alloy materials, applied in the fields of material science and condensed matter physics, can solve the problems of inability to meet the cooling conditions of amorphous alloys, limited amorphous forming ability, etc., and achieve good amorphous forming ability and glass forming ability. , the effect of low production cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
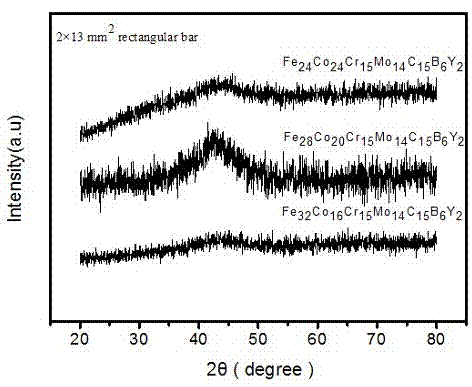
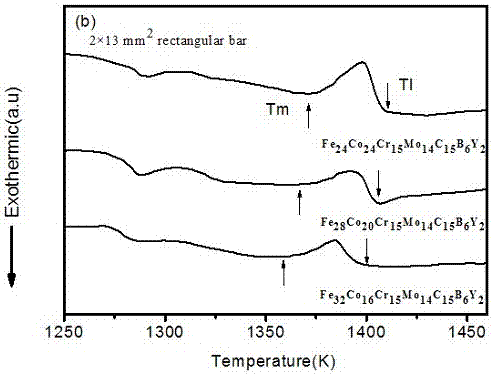
[0011] Embodiment 1. The iron-based amorphous alloy of this embodiment is composed of Fe, Co, Cr, Mo, C, B, and Y, and Fe, Co, Cr, Mo, C, B, and Y is arc-melted in the argon gas adsorbed by titanium according to the required atomic ratio to make it evenly mixed, sucked and cooled to obtain a plate-shaped amorphous alloy Fe with a thickness of 2 mm and a width of 13 mm. 24 co 24 Cr 15 Mo 14 C 15 B 6 Y 2 . The X-ray diffraction pattern confirmed a completely amorphous state. The glass transition temperature (T g =842K) initial crystallization temperature T x =904K and melting point T m =1371K. The supercooled liquid region is ΔT x =62K, indicating that its ability to form amorphous is very good. The Vicker hardness reaches 1298Hv, and the compressive fracture strength of this alloy is 3200Mpa.
Embodiment 2
[0012] Embodiment two, the technical scheme is consistent with embodiment one, and the composition of the iron-based bulk amorphous alloy prepared is Fe 28 co 20 Cr 15 Mo 14 C 15 B 6 Y 2 Fe in this embodiment 28 co 20 Cr 15 Mo 14 C 15 B 6 Y 2 Amorphous alloys have a high glass-forming ability, and this alloy is characterized by a glass transition temperature of T g =860K, the crystallization temperature is T x =900K, its melting point is T m =1366K, the supercooled liquid phase region is 40K, and its amorphous formation ability is better than that of the bulk amorphous alloy prepared in implementation 1. The Vicker hardness reaches 1280Hv, and the compressive fracture strength of this alloy is 2800MPa.
Embodiment 3
[0013] Embodiment three, technical scheme as embodiment one, the bulk amorphous component that prepares is Fe 32 co 16 Cr 15 Mo 14 C 15 B 6 Y 2 , the alloy replaces part of the Co of the implementation 1 scheme with Fe to obtain Fe 32 co 16 Cr 15 Mo 14 C 15 B 6 Y 2 Amorphous alloys have high glass-forming ability, the alloy reduces cost by replacing the more expensive metal Co with cheaper iron, and the alloy has good thermal stability. The glass transition temperature T of the alloy g 854K, crystallization temperature Tx The temperature is 893K, the melting point Tm is 1358K, the supercooled liquid zone width of the alloy is 39K, the Vickers hardness is 1269Hv, and the compressive fracture strength is 2750Mpa.
[0014] During implementation, the prepared material is represented by the following formula: Fe 24+x co 24-x Cr 15 Mo 14 C 15 B 6 Y 2 (0≤x≤10), its thermodynamic parameter data is recorded in Table 1; the cooling rate of the present invention is r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com