Novel intrauterine sustained control release drug delivery system and preparation method thereof
A drug delivery system and controlled-release drug technology, applied in the field of a new type of intrauterine sustained and controlled-release drug delivery system and its preparation, can solve the problems of uterine injury, copper woman injury, unreasonable shape of the drug delivery system, etc., and restore reproductive function , to avoid bleeding, the effect of high contraceptive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
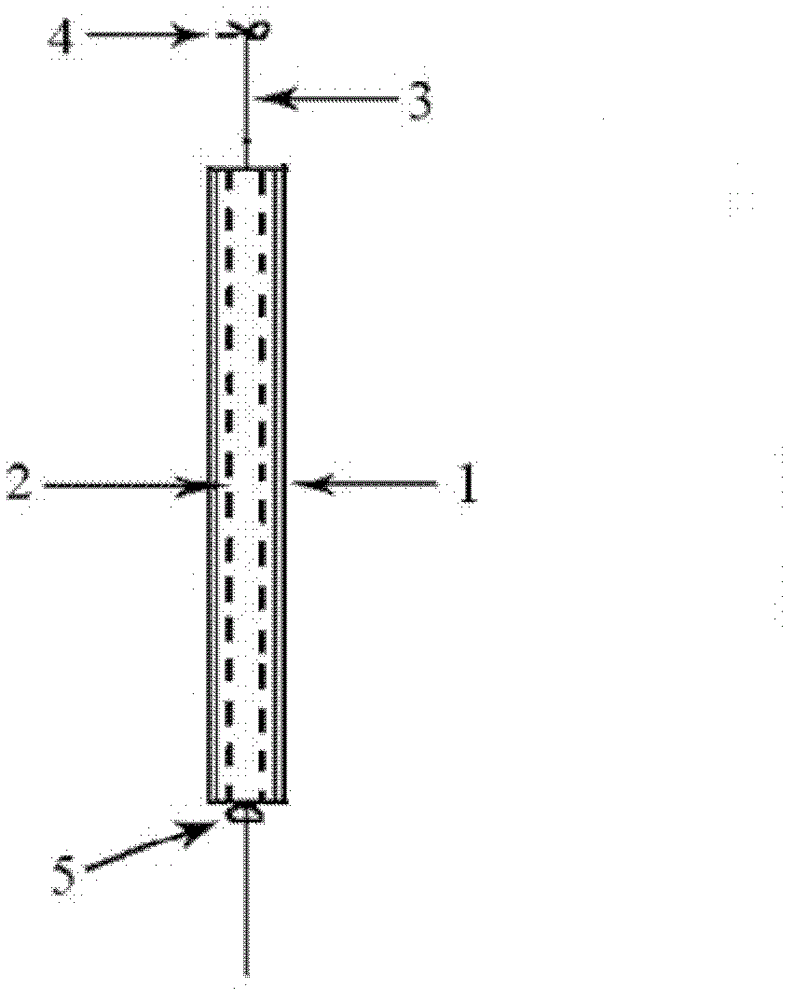
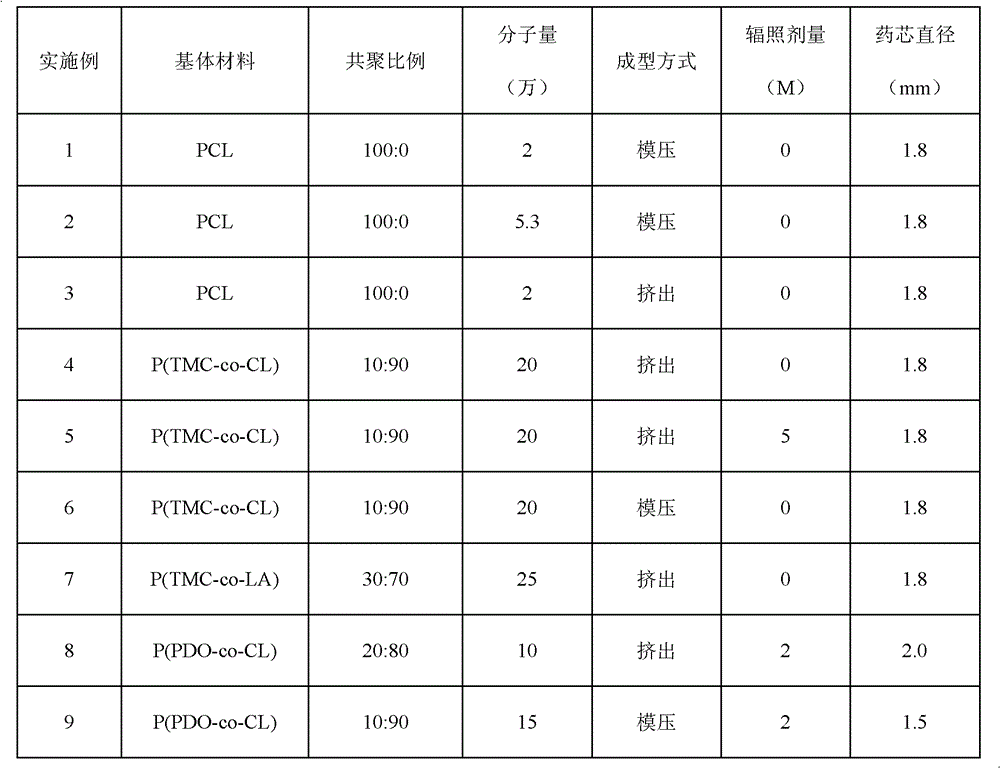
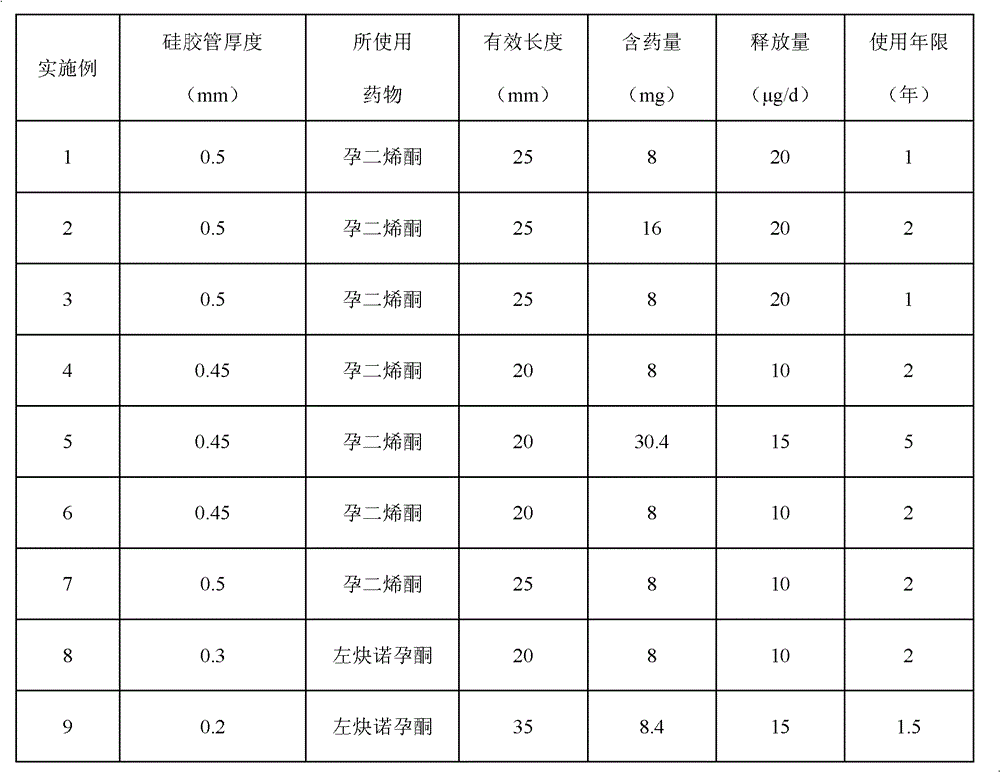
[0044] 1. Set the molecular weight to 2×10 4 g / mol polycaprolactone and gestodene crystalline powder (mass ratio: 100:4) were stirred in an internal mixer at 70°C for 5 minutes to form a premix, and then put into the mold of the pressing rod, and the mold was placed On a flat vulcanizing machine, preheat at 80°C for 2 minutes, then pressurize at 5Mpa for 1 minute, take it out, and cold press it for 2 minutes, and finally remove the mold to obtain a gestodene-containing drug core with a diameter of 1.8mm, and make an effective length of gestodene-containing Intrauterine drug delivery system of enone 8mg / 25mm.
[0045] 2. Put the drug core and polypropylene tail wire prepared in step 1 into a silicone rubber slow-release tube with a length of 30 mm, an outer diameter of 3.0 mm, and a wall thickness of 0.5 mm.
[0046] 3. The two ends of the silicone rubber sustained and controlled release tube are sealed with a silicone rubber adhesive to obtain an intrauterine drug delivery sy...
Embodiment 2
[0048] The difference from Example 1 is that the mass ratio of polycaprolactone to gestodene crystalline powder is 100:8, so that the drug core contains gestodene 16mg / 25mm, and the molecular weight of polycaprolactone used is 5.3× 10 4 g / mol, the release amount is 20μg / d, and the service life is at least 2 years.
Embodiment 3
[0050] The difference from Example 1 is that the preparation method of the drug core containing gestodene is extrusion molding, and the molecular weight is 2×10 4 The g / mol polycaprolactone and gestodene crystalline powder (mass ratio is 100:4) were stirred at 70°C for 5 min in an internal mixer to form a premix, and then extruded on a micro precision extruder with a diameter of 1.8mm drug core containing gestodene, the screw speed is 20r / min, the traction speed is 20cm / min, the melt pump speed is 5r / min, and the air flow rate is 10ml / min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com