Monoclonal antibodies against the pbp2-a protein and homologous sequences for the treatment of infections by and immunodiagnostics of bacteria of the firmicutes phylum
A monoclonal antibody and sequence technology, applied in the direction of antibodies, anti-enzyme immunoglobulins, anti-bacterial immunoglobulins, etc., can solve the problem that vaccines cannot produce protective antibodies in time for bacterial infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] 4. Preparation of Monoclonal Antibody
[0068] Lymphocytes taken from the spleen were fused with SP2 / 0-Ag14 myeloma cells (ATCC 1581) according to the record of monoclonal antibody production in Current Protocols in Immunology (22), using polyethylene glycol fusion, at 37°C, 10 %CO 2 cultured in hypoxanthine-aminopterin-thymus medium. Synthetic hybridomas were evaluated after 14 days by ELISA assay using purified recombinant protein as antigen as described below. The best hybridoma cells were used for cloning, the best clones were selected by ELISA, and these clones were then stored in liquid nitrogen.
[0069] 5. Enzyme immunoassay—ELISA
[0070] Maxisorb 96-well plastic plates were coated with recombinant protein (PBP2a fragment) in carbonate / bicarbonate buffer at 500 ng / well and incubated overnight at 4°C. On the following day, the plate was washed three times with PBS containing 0.05% Tween 20 and blocked for two hours at 37°C in PBS and 5% skim milk. Samples f...
Embodiment 2
[0122] 1 Acquisition of mouse anti-PBP2a monoclonal antibody
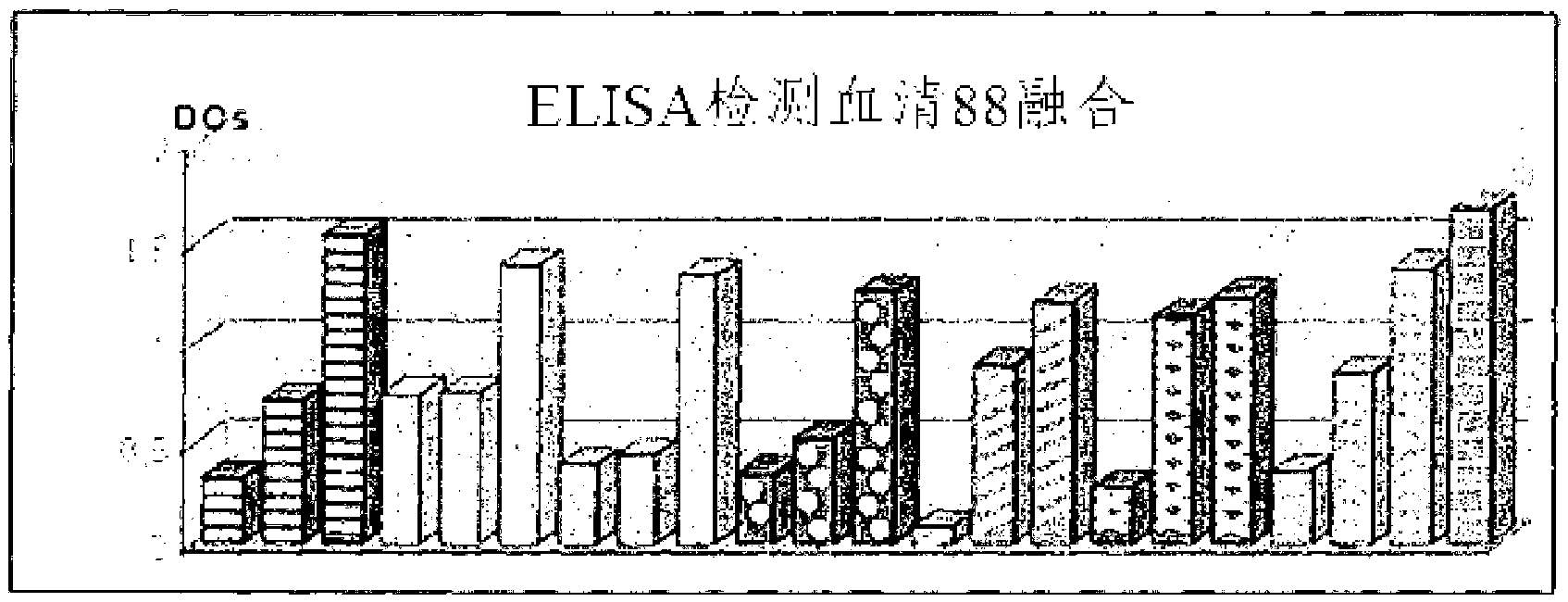
[0123] A group of animals was immunized according to the previously described immunization protocol. The results evaluated by ELISA are described in figure 1 middle.
[0124] After the degree of fusion (fusion of 90-LATAM), suspensions from 96-well plates (hybridomas) were analyzed via ELISA detection. From this total, the five best samples are selected to expand the cells (clones). Then, again, the resulting suspension was analyzed by the ELISA method. Results obtained by ELISA were confirmed by immunoblotting against purified recombinant protein (PBP2a) and confirming positive samples. The final results are in Table I.
[0125] figure 1 Results obtained by enzyme immunoassay (ELISA) for the production of antibodies against PBP2a in the sera of vaccinated animals are shown. Each jumping wave corresponds to a 1:100 dilution of sera from vaccinated animals. Positive control sera are angled columns [ ]. ...
Embodiment 3
[0241] A second study using the CEB MRSA strain, using the same method as described in Example 1, section 7.2, was assisted by two pulses of vortex agitation, 15 seconds each, after each wash to help grapevine aureus Dissociation of cocci clumps and increased exposure of PBP2a to antibodies.
[0242] In control samples (i pure bacteria, not exposed to mAb; and ii pure bacteria plus FITC or PE marker) and mAb-treated samples, after labeling with PE or FITC for analysis, Detection markers FITC (fluorescein isothiocyanate) and PE (phycoerythrin). Read in linear mode in a FACsalibur instrument.
[0243] Figure 10 is a graph of flow cytometry analysis of MRSA samples in the presence of FITC-labeled anti-PBP2a antibody. Curve (x) corresponds to unlabeled samples, curve (y) corresponds to labeled samples.
[0244] The results obtained showed that approximately 22% of the labeled population was instrumentally detected, confirming the recognition of the anti-PBP2a antibody for the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com