O-nitro aryl methoxycamptothecine anoxic activated prodrug used for antitumor drug
A nitroarylmethoxy camptotheca, hypoxia-activated prodrug technology, applied in the field of anti-tumor drugs, can solve the problems of unstable plasma metabolism, toxic side effects, insoluble, etc., and achieves improved water solubility and stability, low Toxic side effects, highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
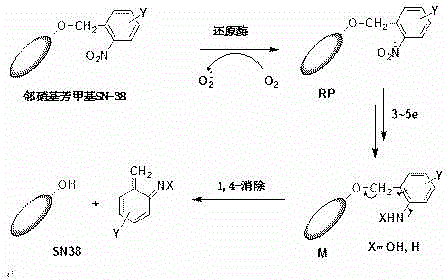
[0063] Embodiment 1. o-nitrobenzyl SN-38 and its preparation method
[0064] 1) The chemical name of o-nitrobenzyl SN-38 is:
[0065] (4S)-4,11-Diethyl-4-hydroxy-9-(2-nitrobenzyloxy)-1H-pyrano[3',4':6,7]indoleazino[1 ,2-b] quinoline-3,14(4H,12H)-dione
[0066] The chemical structural formula is:
[0067]
[0068] 2) The preferred preparation method of o-nitrobenzyl SN-38:
[0069] Prepared by reaction of o-nitrobenzyl bromide and SN-38
[0070] Dissolve 4.75 g of o-nitrobenzyl bromide and 3.92 g of SN-38 in 40 ml of N,N-dimethylformamide, add 1.38 g of potassium carbonate at room temperature, raise the temperature to 85°C after the addition, and stir for 5 hours , the system was cooled to room temperature. Add 200 ml of dichloromethane and 200 ml of water, separate the organic phase, and extract the aqueous layer with dichloromethane (50 ml × 3); combine the organic phases and dry them with anhydrous sodium sulfate, remove the solvent under reduced pressure, and obtai...
Embodiment 2
[0076] Example 2. The application effect of o-nitrobenzyl SN-38 and its comparison with the standard drug irinotecan of camptothecin derivatives
[0077] 1) Identification of anticancer activity of o-nitrobenzyl SN-38 and comparative analysis with irinotecan:
[0078] image 3 It shows the growth of subcutaneous liver cancer HepG2 tumor in nude mice after treatment with o-nitrobenzyl SN-38 and irinotecan, and the comparative analysis with the growth of tumor in the control group.
[0079] 1 × 106 human liver cancer HepG2 cells in logarithmic growth phase were subcutaneously injected into the left flank of 6-week-old female Balb / c nude mice. When the tumor grew to 100 mm3 (day 0), the animals were randomly divided into three groups, namely the control group, the irinotecan group and the o-nitrobenzyl SN-38 group, and were given intraperitoneal injection of normal saline, irinotecan (50mg / kg, sorbitol / lactic acid buffer [45 mg / ml sorbitol / 0.9 mg / ml lactic acid] and o-nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com