Sulfhydryl compound containing beta-ester groups and acetyl-transferred synthesis method thereof
A technology for transfer of mercapto compounds and acetyl groups, applied in organic chemistry, thiol preparation, etc., can solve problems such as low reaction rate, low overall yield, and narrow application range, and achieve simple process, high yield, and wide application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
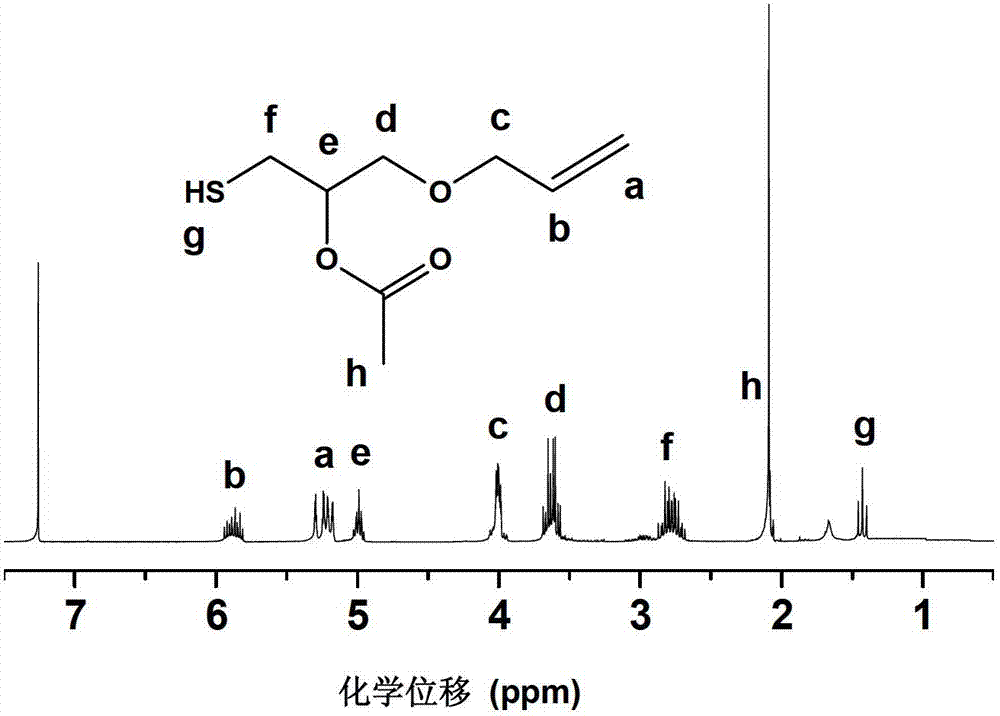
[0041] Under the protection of nitrogen, add 1 mole of allyl glycidyl ether, 5 moles of methanol, 0.5 moles of triethylamine, and 1.01 moles of monothioacetic acid to the reactor successively, react at -10°C for 24 hours, and wash with dilute hydrochloric acid , evaporated to remove the solvent, and then evaporated under reduced pressure to remove excess monothioacetic acid. The product yield obtained in this example was 97%. figure 1 It is the mercapto compound containing β-ester group and vinyl group synthesized with allyl glycidyl ether and thioacetic acid in this embodiment 1 The H NMR spectrum shows significant signals for thiol protons (1.42 ppm) and vinyl hydrocarbon protons (5.24 and 5.85 ppm). figure 2 It is the mercapto compound containing β-ester group and vinyl group synthesized with allyl glycidyl ether and thioacetic acid in this embodiment 13 The C NMR spectrum shows obvious signals of the carbon atom connected to the mercapto group (25.0ppm), the signal of t...
Embodiment 2
[0043] Under nitrogen protection, 1 mole of propargyl glycidyl ether, 100 moles of ethanol, 2 moles of tetramethylethylenediamine, and 1.5 moles of monothioacetic acid were successively added to the reactor, and reacted at 30°C for 1 hour. Washed with hydrochloric acid, evaporated to remove the solvent, and evaporated under reduced pressure to remove excess monothioacetic acid. The product yield obtained in this example was 98%. image 3 It is the synthesis of β-ester group and alkynyl-containing mercapto compound with propargyl glycidyl ether and thioacetic acid in this embodiment 1 H NMR spectrum, showing a clear signal of sulfhydryl protons (1.42ppm) and alkyne protons (2.45ppm). Figure 4 It is the synthesis of β-ester group and alkynyl-containing mercapto compound with propargyl glycidyl ether and thioacetic acid in this embodiment 13 The C NMR spectrum shows obvious signals of the carbon atom connected to the mercapto group (25.0ppm), the signal of the carbon atom of t...
Embodiment 3
[0045] Under nitrogen protection, sequentially add 1 mole of propargyl glycidyl ether, 10 moles of chloroform, 1 mole of pyridine, and 1.1 moles of monothiobenzoic acid into the reactor, react at 10°C for 1.5 hours, and wash with dilute hydrochloric acid. The solvent was removed by evaporation and excess monothiobenzoic acid was removed by column chromatography. The product yield obtained in this example was 98%. Similarly, image 3 It is the synthesis of β-ester group and alkynyl-containing mercapto compound with propargyl glycidyl ether and thioacetic acid in this embodiment 1 H NMR spectrum, showing a clear signal of sulfhydryl protons (1.42ppm) and alkyne protons (2.45ppm). Figure 4 It is the synthesis of β-ester group and alkynyl-containing mercapto compound with propargyl glycidyl ether and thioacetic acid in this embodiment 13 The C NMR spectrum shows obvious signals of the carbon atom connected to the mercapto group (25.0ppm), the signal of the carbon atom of the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com