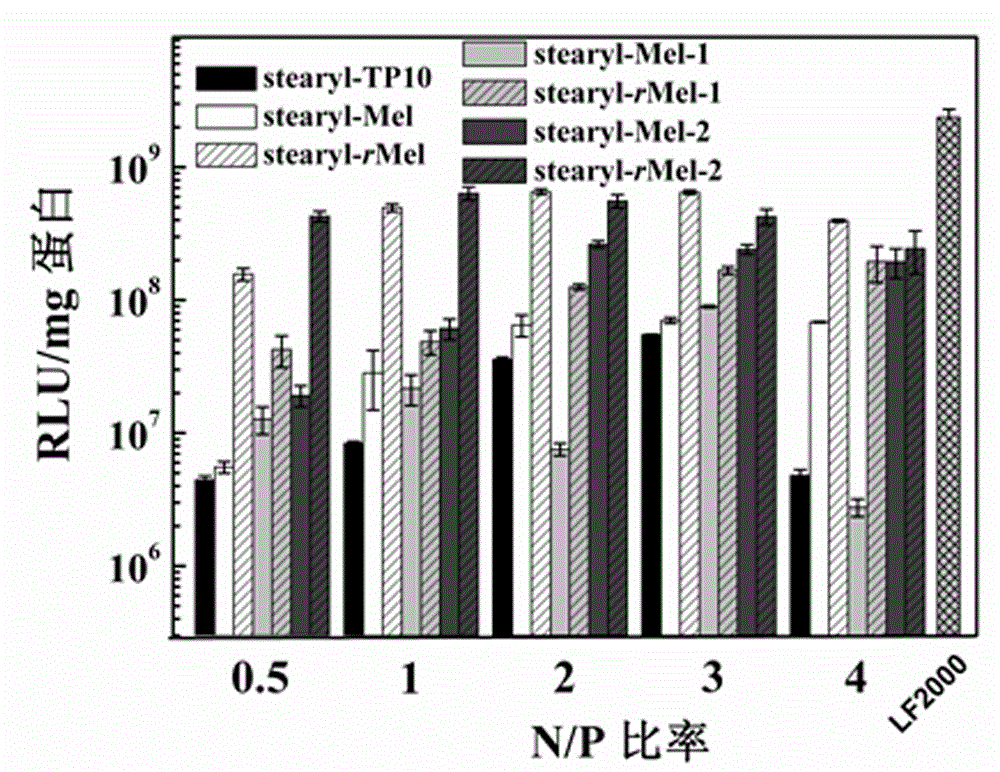
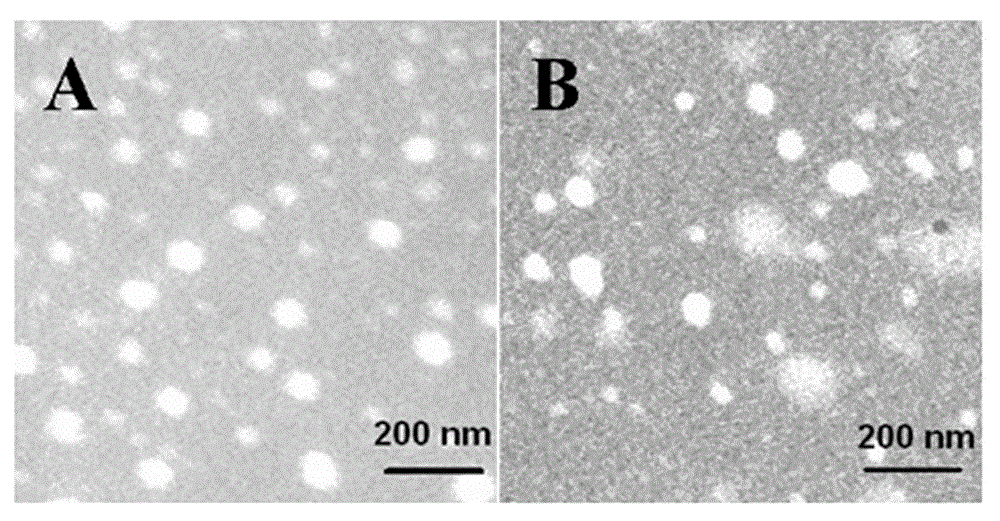
Non-viral gene vector constructed on basis of N-terminal octadecane acylated antibacterial peptide
A gene carrier and antimicrobial peptide technology, applied in the field of non-viral peptide carriers, can solve the problems of low toxicity, etc., and achieve the effects of easy biodegradation, lower application cost, and simple design and synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthesis of stearyl-Mel
[0046] a. Resin pretreatment: Add Rink-Amide-MBHA resin with an amino molar mass of 0.2 mmol into the reactor, add 10 mL of dichloromethane and stir for 30 min to fully swell the resin, and then drain the solvent under reduced pressure.
[0047] b. Deprotection of F-moc: add 10~15mL deprotection reagent into the reactor, stir for 2min, then drain, repeat 4 times to completely remove the Fmoc group, and finally wash with DMF to remove the deprotection reagent; deprotection The reagent is piperidine / DMF=1:4 (V / V).
[0048] c. Condensation: 0.6~0.8 mol Fmoc group-protected amino acid, N-hydroxybenzotriazole, O-benzotriazole-N,N,N',N'-tetramethylurea-hexa Dissolve fluorophosphate in 3~5mL DMF, then add 1.2~1.6 mol diisopropylethylamine and mix to obtain a mixed solution, then put it into the reactor, stir and react for 40~60min; the whole reaction process is protected by argon , the degree of reaction was detected by the indene...
Embodiment 2
[0054] Example 2: stearyl- r Synthesis of Mel
[0055] d. Extension of the peptide chain: Repeat steps b and c in the order of QQRKRKRKIWSILAPLGTTLVKLVAGIG until the peptide grafting is completed. Other steps are the same as in Example 1. The amino acid sequence of the obtained analogue is opposite to that of stearyl-Mel.
[0056] The molecular weight of the product prepared by the above method characterized by ESI-MS is shown in Table 1.
Embodiment 3
[0057] Embodiment 3: the synthesis of stearyl-Mel-1
[0058] d. Extension of the peptide chain: Repeat steps b and c in the order of GIGAVLKVLTTGLPALISWRRRRRRRR until the peptide grafting is completed. Other steps are the same as in Example 1. The sequence of the obtained analog was that the IKRKRQQ sequence at the end of stearyl-Mel was replaced by RRRRRRR.
[0059] The molecular weight of the product prepared by the above method characterized by ESI-MS is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com