Recombinant human nerve growth factor purifying method based on CHO cell expression system
A growth factor and expression system technology, applied in the field of extracting recombinant human nerve growth factor from CHO cell expression supernatant, can solve the problems of protein yield, activity and time loss, high cost of CHO cell expression system, and high probability of protein contamination. , to achieve the effect of good biomolecule binding ability, convenient lyophilization, and reducing the probability of protein contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
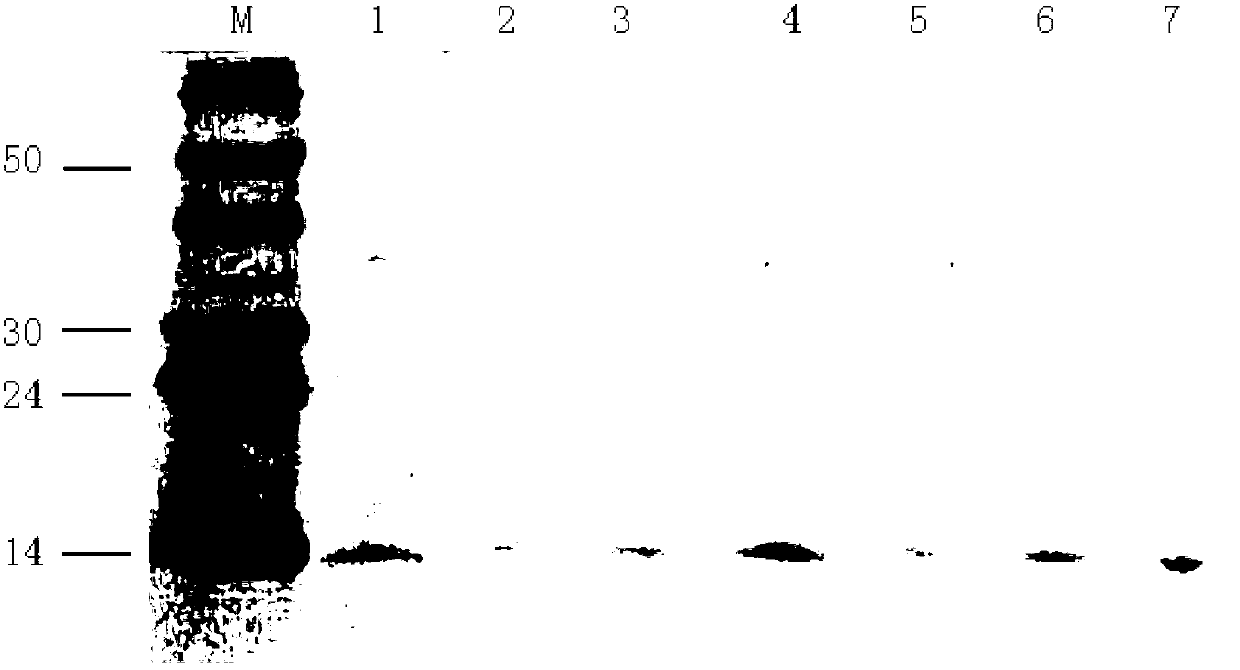
[0044] Embodiment: a kind of purification method of the recombinant human nerve growth factor based on CHO cell expression system comprises the following steps:
[0045] 1. Cell supernatant treatment
[0046] Take the culture medium of CHO cells highly expressing recombinant human nerve growth factor harvested on a shaker for 7-8 days, first centrifuge at 2000rpm for 10min to remove live cells, then centrifuge the supernatant at 8000rpm for 20min to remove cell debris, and pass through the supernatant after centrifugation for 0.22 μm filter membrane to remove impurities and bacteria, then adjust the pH to 7.0 with disodium hydrogen phosphate, and set aside.
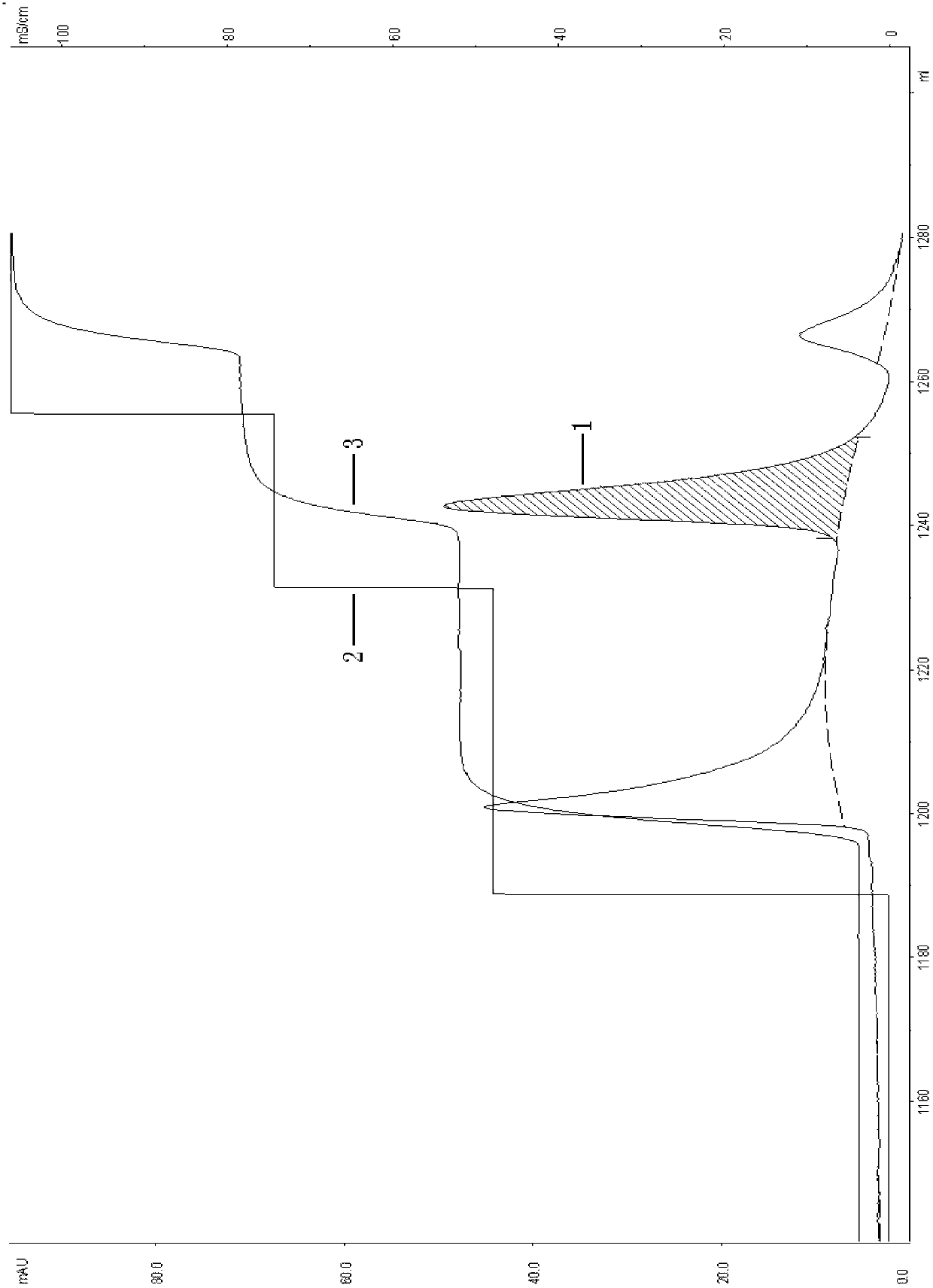
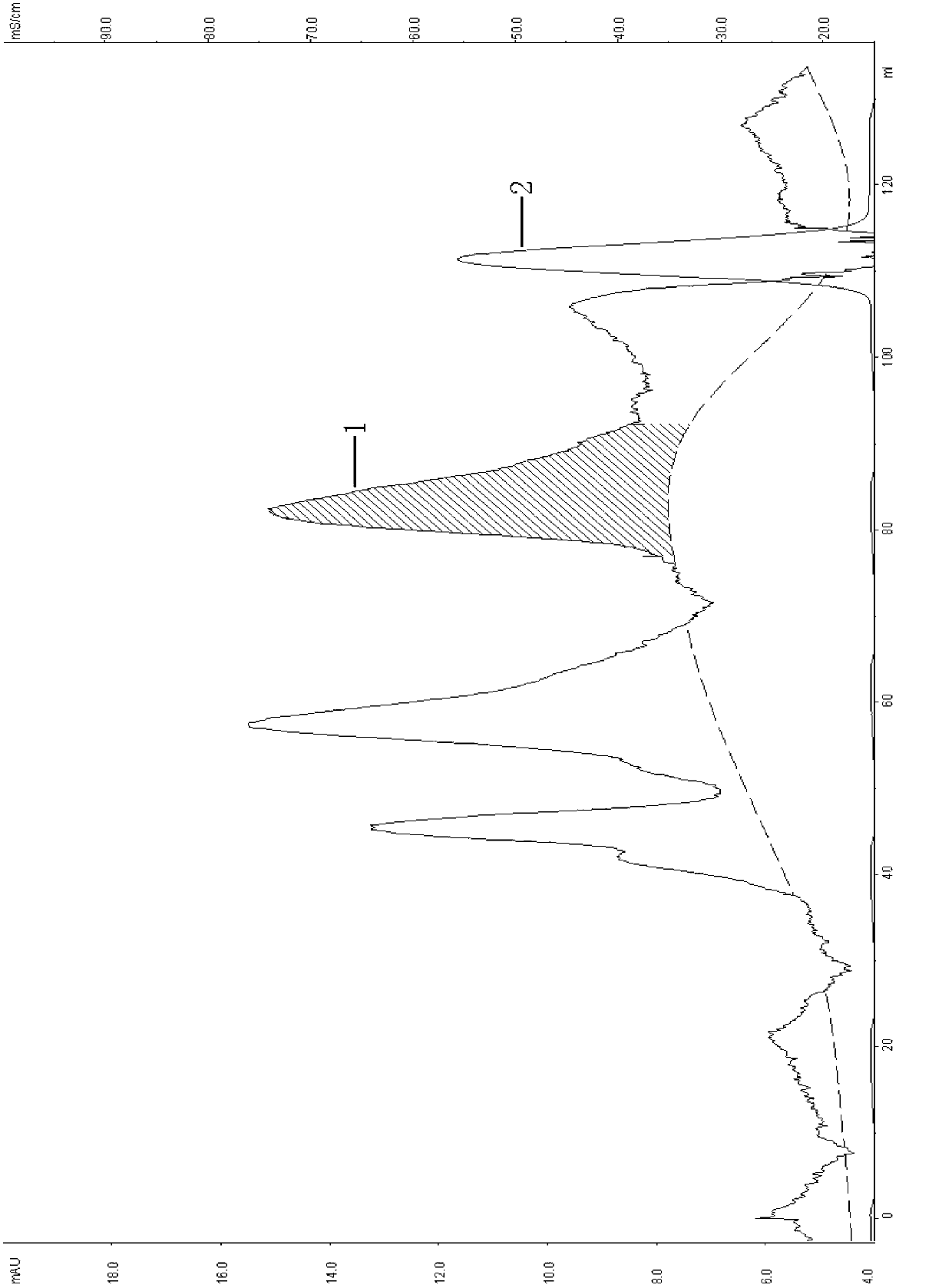
[0047] 2, EMD SO 3 - (M) Chromatography
[0048] Load the cell supernatant after passing through the membrane at pH 7.0, and equilibrate with 20mmol / L PB buffer EMD SO 3 - (M) Chromatographic column, the flow rate is 2ml / min. After loading the sample, use 20mmol / L PB buffer containing 0.45mol / L NaCl, pH7.0 to was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com