New crystal form of penehyclidine hydrochloride and preparation method of new crystal form
A technology for penehyclidine hydrochloride and crystal form, applied in the field of new crystal forms of medicines, can solve the problem of no penehyclidine hydrochloride, etc., and achieve the effects of good stability, guarantee of clinical efficacy, and avoidance of drug impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
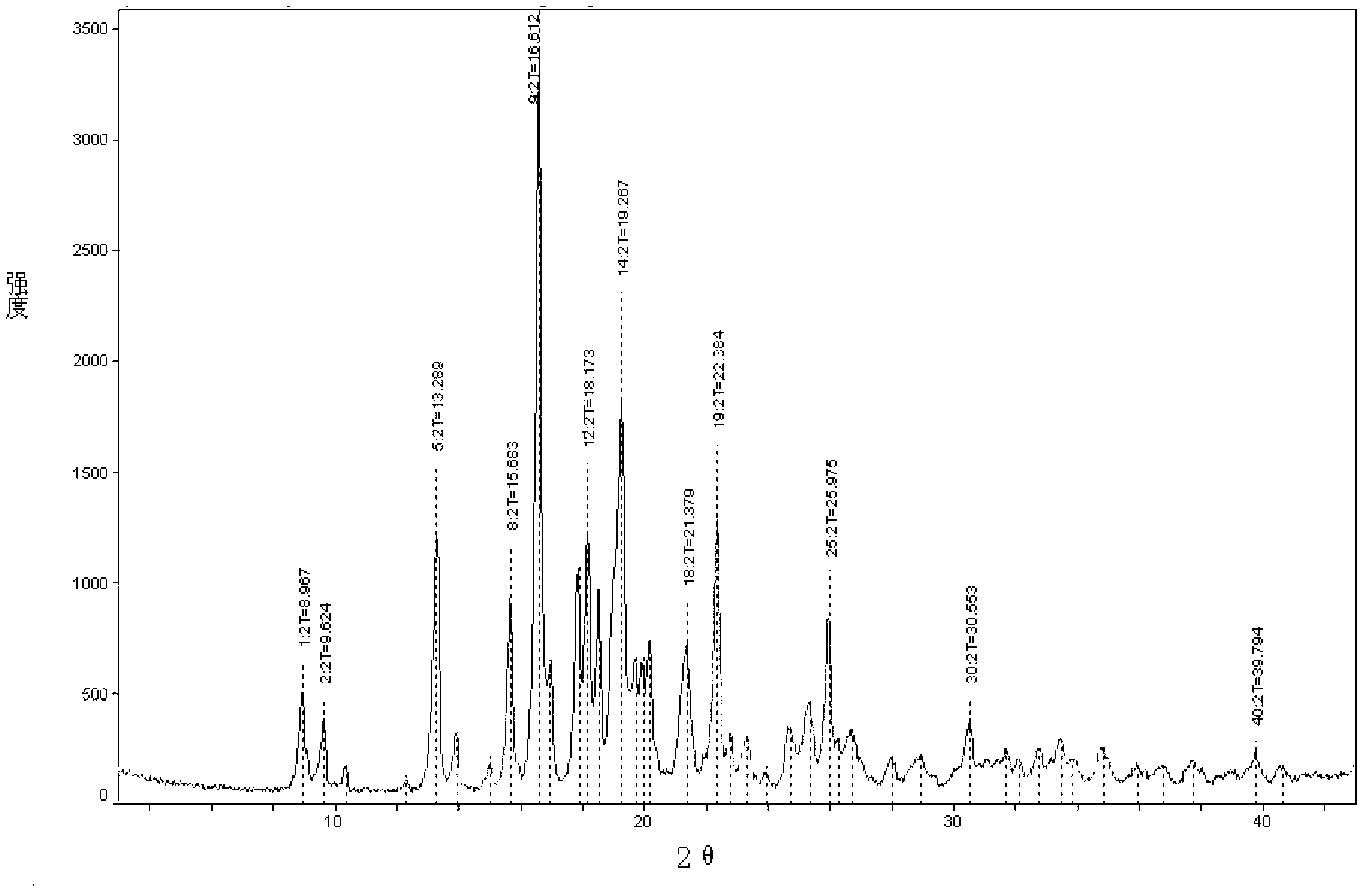
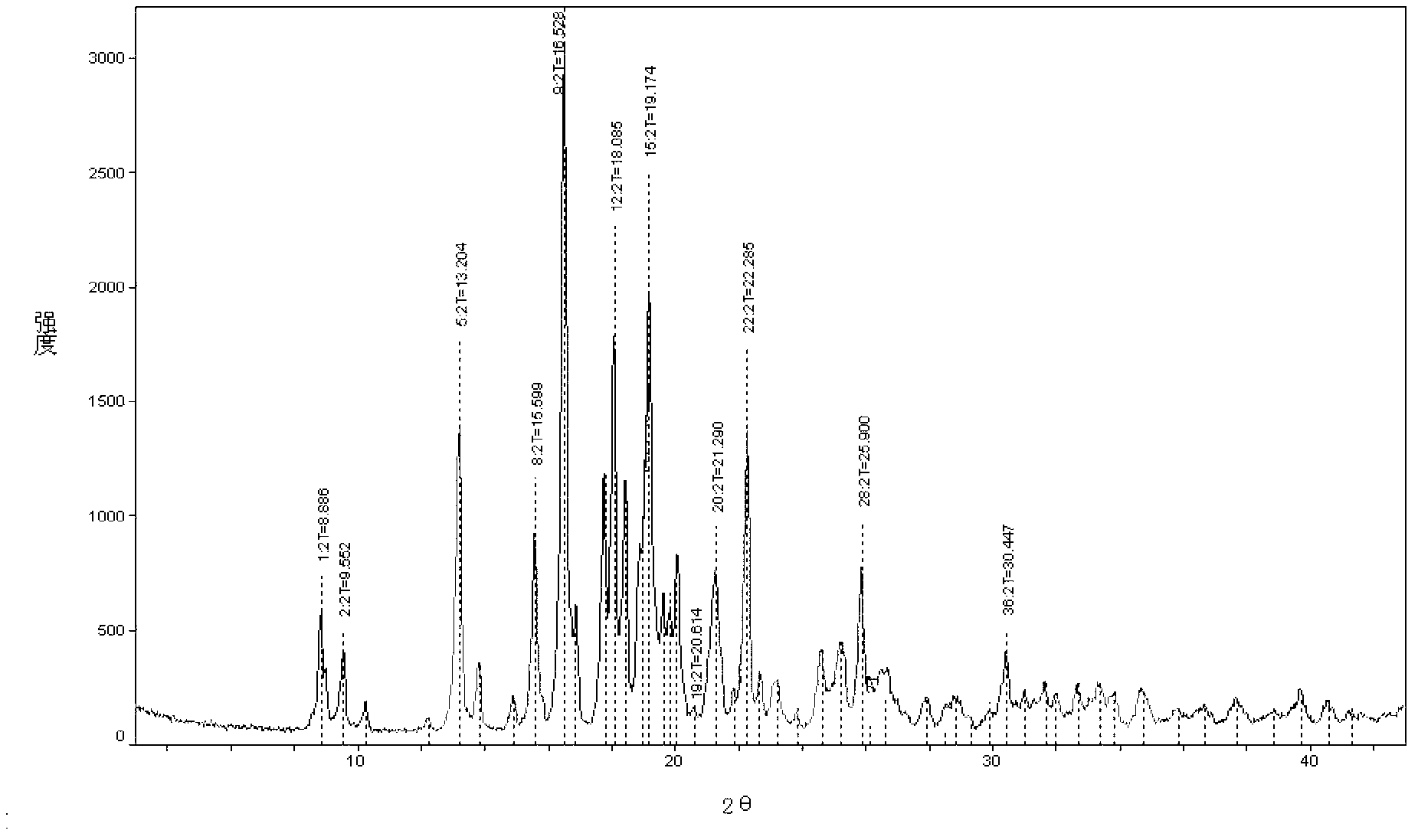
Image
Examples
Embodiment 1
[0030] Embodiment 1 Preparation of penhyclidine hydrochloride crystal form of the present invention
[0031] Take 49.5 g of penehyclidine hydrochloride, add 150 ml of isopropanol to reflux to dissolve, then add 200 ml of petroleum ether while it is hot and let it cool down naturally.
[0032] Wherein, raw material penhyclidine hydrochloride is prepared by the following method:
[0033] Add 350ml of dimethyl sulfoxide to a 1L three-necked flask, then add 25.4g (0.20mol) of 3-quinuclidinol and 8.4g (0.21mol) of 60% sodium hydride, stir at room temperature for two hours until no bubbles are generated, then drop Add 39.0g (0.201mol) of 1-phenyl-1-cyclopentyl oxirane, react at 50°C for 30 hours after the addition is complete, stop the reaction, remove impurities after acid and alkali treatment, and then use 500ml ethyl acetate Extract, wash with brine until neutral, concentrate and add 400ml ether hydrochloric acid solution to obtain 49.5g of penhyclidine hydrochloride.
[0034] ...
Embodiment 2
[0035] Embodiment 2 Preparation of penhyclidine hydrochloride crystal form of the present invention
[0036] Add 600ml of dimethyl sulfoxide to a 1L three-necked flask, then add 50.8g (0.40mol) of 3-quinuclidinol and 17g (0.42mol) of 60% sodium hydride, stir at room temperature for two hours until no bubbles are generated, and then add dropwise 80g (0.41mol) of 1-phenyl-1-cyclopentyl oxirane, react at 35°C for 45 hours after the addition is complete, stop the reaction, treat with acid and alkali, extract with 800ml ethyl acetate, wash with brine until neutral, After concentrating, add 800ml of ethyl acetate hydrochloric acid solution to form hydrochloride, and obtain 101.8g of solid, add 300ml of isopropanol to reflux to dissolve, then add 300ml of ethyl acetate while it is hot, and naturally cool to precipitate crystals, in order to ensure complete crystallization, then lower the temperature to Further crystallization was carried out at 0-5°C. After the crystallization was co...
Embodiment 3
[0037] Embodiment 3 Preparation of penhyclidine hydrochloride crystal form of the present invention
[0038] Add 500ml of dimethyl sulfoxide into a 1L three-necked flask, then add 25.4g (0.20mol) of 3-quinuclidinol and 8.4g (0.21mol) of 60% sodium hydride, stir at room temperature for two hours until no bubbles are generated, then drop Add 39.0g (0.201mol) of 1-phenyl-1-cyclopentyl oxirane, react at 40°C for 40 hours after the addition is complete, stop the reaction, treat with acid and alkali, extract with 500ml ethyl acetate, wash with brine to medium After concentration, add 100ml of ethanol hydrochloric acid solution, then add 300ml of petroleum ether, after natural cooling, then cool down to 0-5°C to crystallize, and filter to obtain 32.9g of the product, with a yield of 46.74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com