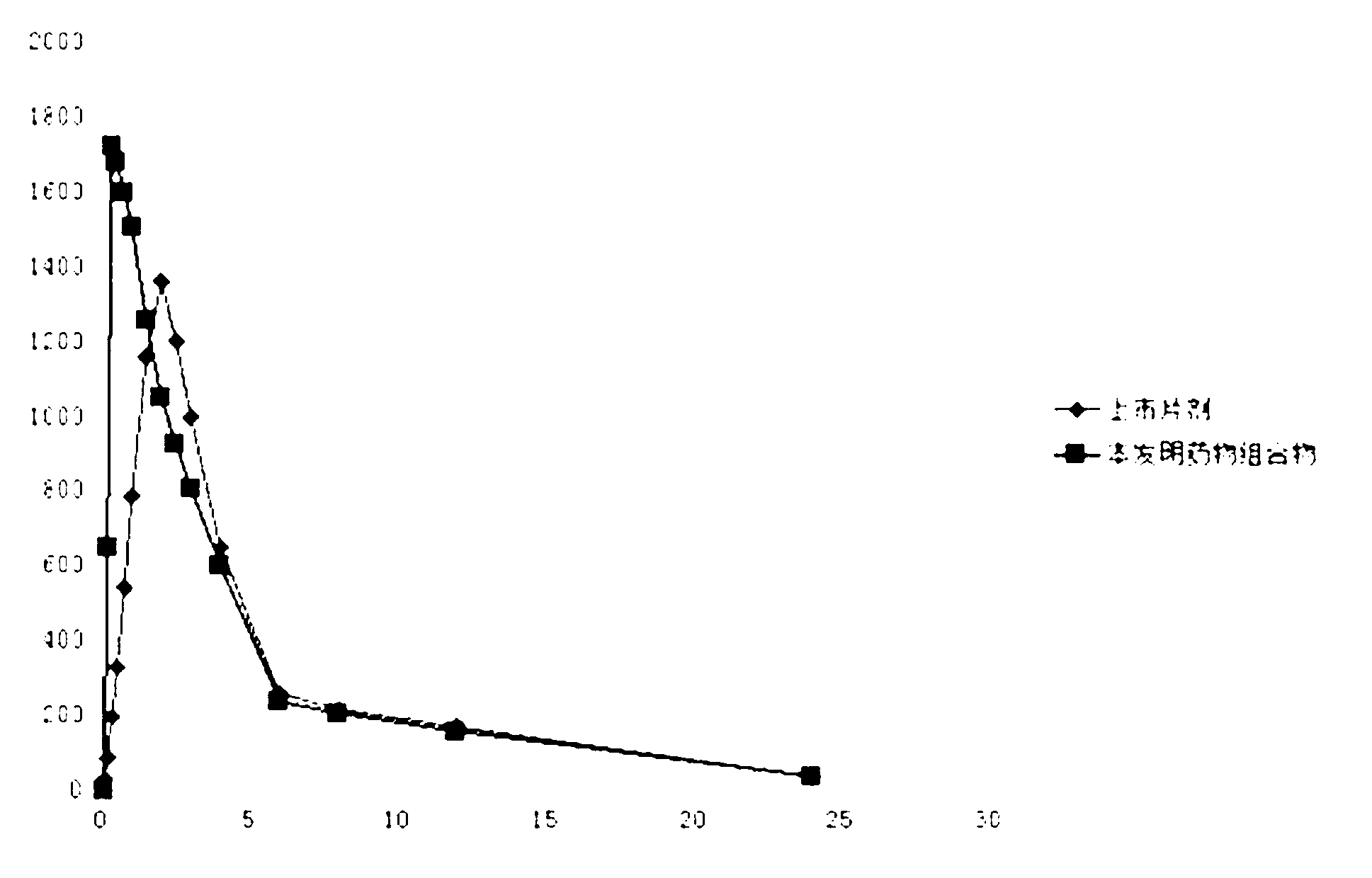
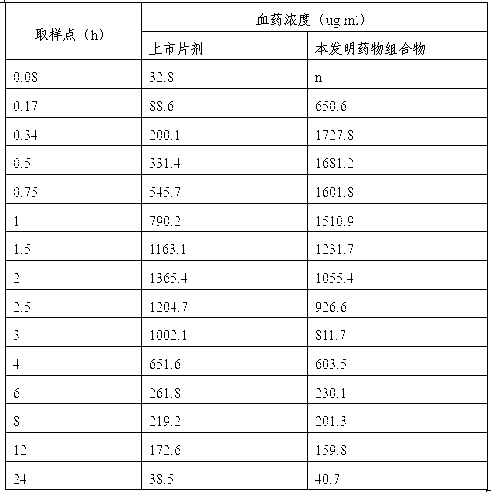
Medicinal composition of pharmaceutically-acceptable salt containing clopidogrel and preparation method thereof
A composition and pharmaceutical technology, applied in the field of medicine, can solve problems such as product quality and clinical curative effect, and achieve the effects of solving crystal form and electrostatic problems, shortening peak time, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 clopidogrel thiohydrogen soft capsules
[0056] The composition of the prescription is as follows: (g)
[0057] Clopidogrel Sulfuric Acid 24.46
[0058] PEG400 842
[0059] 1,2-Propanediol 150
[0060] TW80 8.5
[0061] A total of 1024.96g, made into 1000 soft capsules.
[0062] Preparation process: Weigh the prescribed amount of PEG400, 1,2-propanediol, TW80, add to the dissolving tank, stir and mix evenly at 45-70°C; pass the drug through a 60-mesh sieve, and weigh the prescribed amount of thiohydrogen Clopidogrel (crystal form is not limited), add to the mixed medium, after the drug dissolves, filter and transfer to the liquid medicine tank, keep warm at 30-38°C, connect to a soft capsule machine, press into soft capsules, dry, and divide after passing the quality inspection Packing, labeling, and packaging, the clopidogrel sulfide soft capsules are obtained.
Embodiment 2
[0063] Embodiment 2 Preparation of Clopidogrel Sulfate Soft Capsules
[0064] The composition of the prescription is as follows: (g)
[0065] Clopidogrel Sulfuric Acid 24.46
[0066] PEG200 716
[0067] 1,2-Propanediol 80
[0068] ethanol 7
[0069] HS-15 0.8
[0070] A total of 828.26g, made into 1000 soft capsules.
[0071] Preparation process: Weigh the prescribed amount of PEG200, 1,2-propylene glycol, ethanol, HS-15, add to the dissolution tank, stir at 45-70°C to mix evenly; pass the drug through a 60-mesh sieve, weigh Prescribed amount of clopidogrel sulfide (crystal form is not limited), add to the mixed medium, after the drug dissolves, filter and transfer to the liquid medicine tank, keep warm at 30-38°C, connect to a soft capsule machine, press into soft capsules, and dry , after passing the quality inspection, subpackage, label, and pack to obtain the clopidogrel sulfide soft capsules.
Embodiment 3
[0072] The preparation of embodiment 3 clopidogrel hydrobromide soft capsules
[0073] The composition of the prescription is as follows: (g)
[0074] Clopidogrel hydrobromide 23.80
[0075] PEG600 500
[0076] 1,2-Propanediol 50
[0077] Tween 80 29
[0078] A total of 602.8g, made into 1000 soft capsules.
[0079] Preparation process: Weigh PEG600, 1,2-propanediol, and Tween 80 in the prescribed amount, add them to the dissolving tank, stir at 45-70°C to mix them evenly; pass the drug through a 60-mesh sieve, and weigh the prescribed amount Clopidogrel hydrobromide (crystal form is not limited), added to the mixed medium, after the drug is dissolved, filtered and transferred to the liquid medicine tank, kept at 30-38 ° C, connected to a soft capsule machine, compressed into soft capsules, dried, quality After passing the test, it is subpackaged, labeled, and packaged to obtain the finished product of clopidogrel hydrobromide soft capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com