Loop-line-shaped phosphazene epoxide resin and synthetic method thereof
A nitrile epoxy resin and phosphazene-shaped technology, which is applied in the field of epoxy resin and its synthesis, can solve the problems of unsatisfactory thermal properties and physical and mechanical properties, and achieve the effect of excellent flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
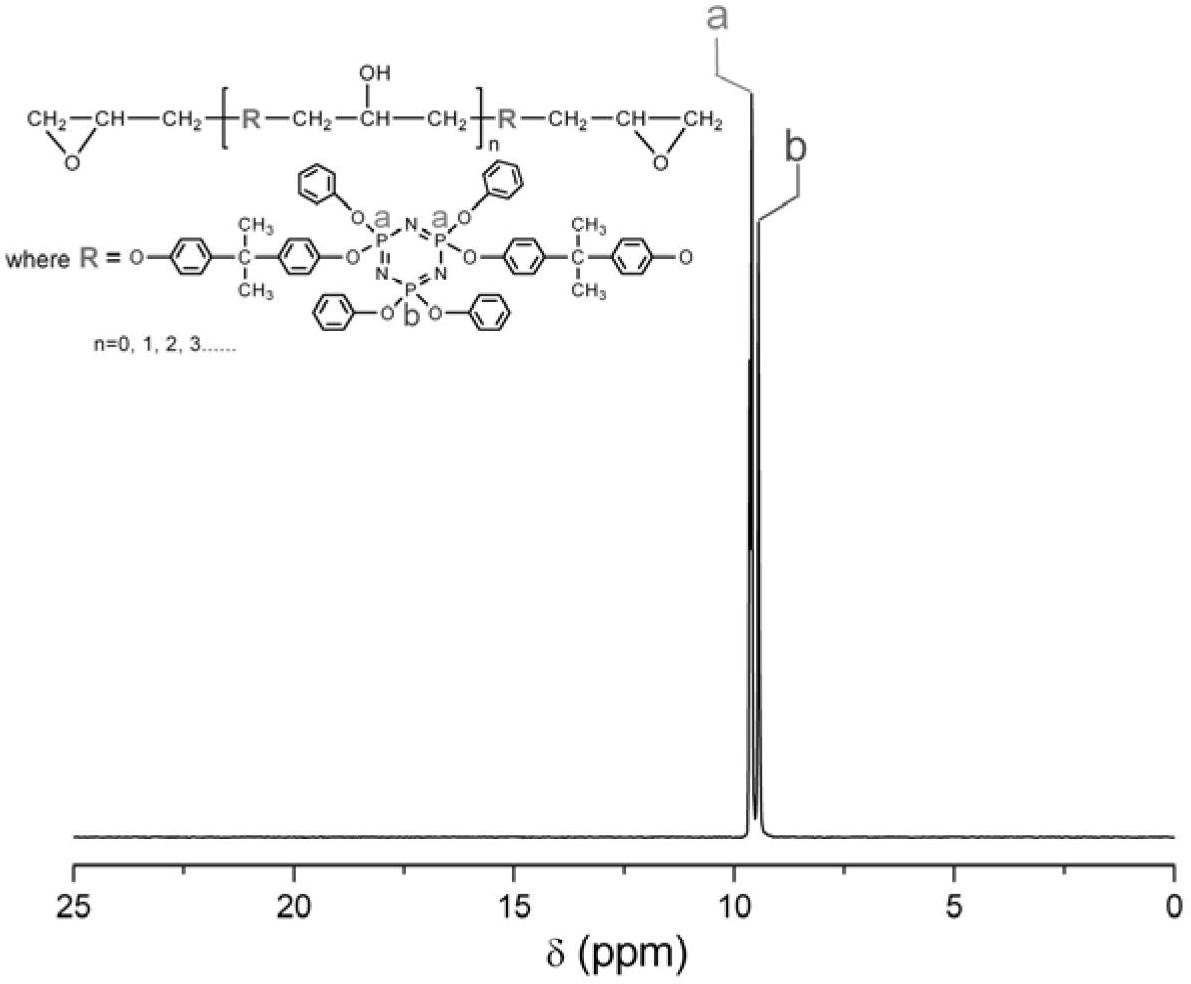
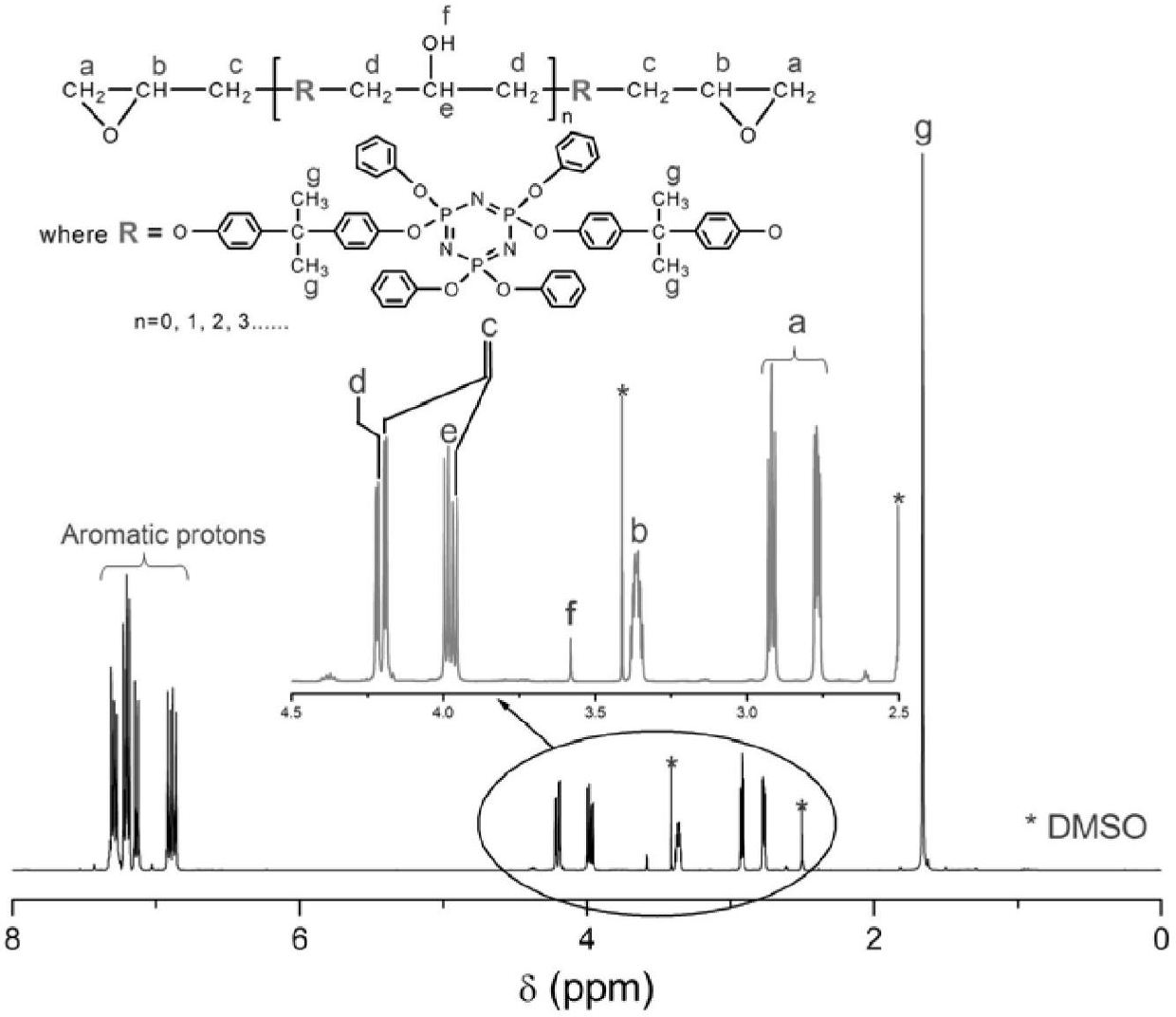
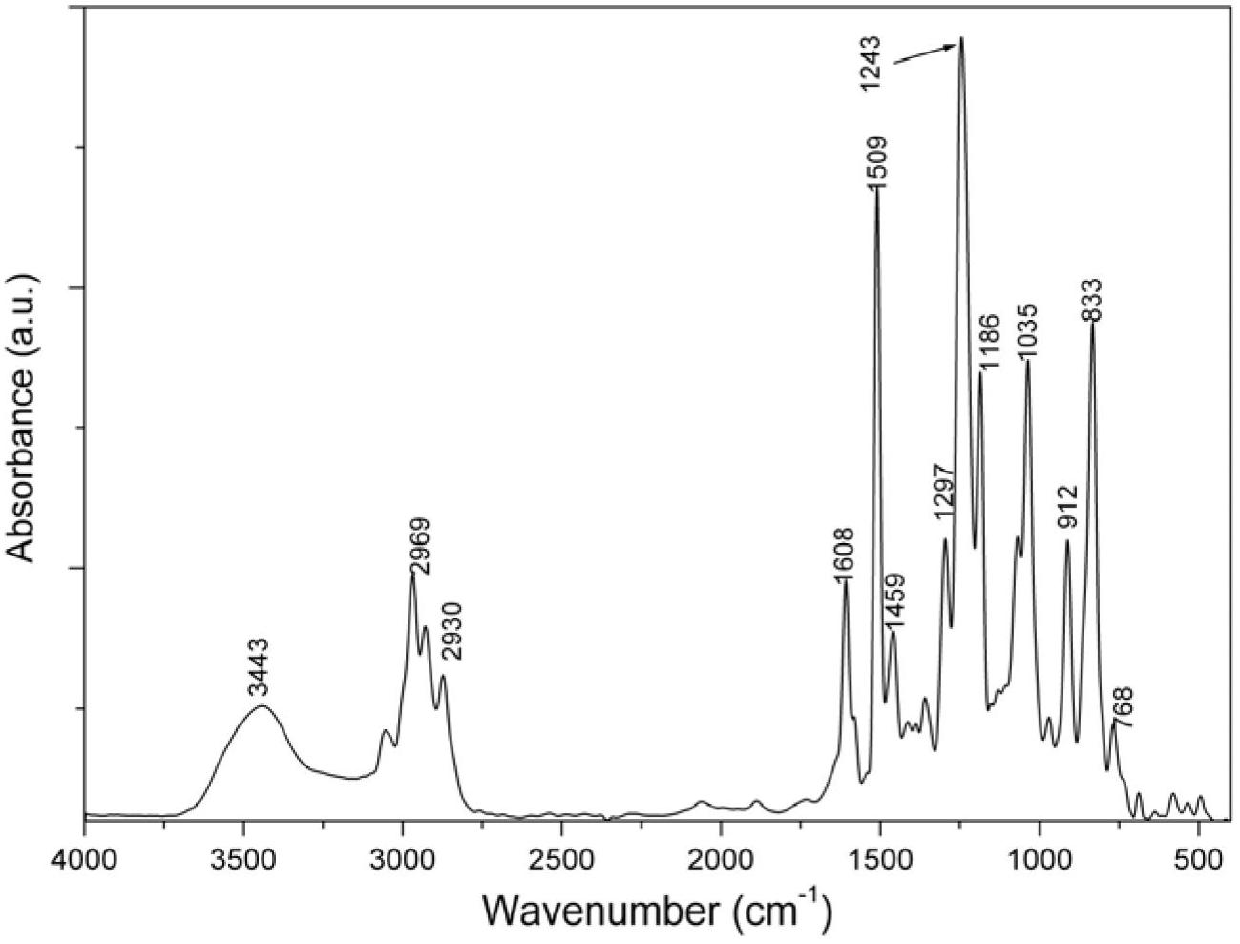
[0042] When the structure of R is C 6 H 4 C(CH 3 ) 2 C 6 H 4 The synthetic process route of the ring linear phosphazene epoxy resin CLPN-EP1 is as follows figure 1 shown.
[0043] The synthetic embodiment of CLPN-EP1 is as follows:
[0044]
[0045] n is an integer of 0-10.
[0046] In step 1, under the protection of nitrogen atmosphere, phenol is dissolved in tetrahydrofuran, then sodium hydride is added, and the reaction is carried out at room temperature for 2 hours to obtain a corresponding reaction mixture solution. The reaction mixture solution was added dropwise to a solution of hexachlorocyclotriphosphazene dissolved in tetrahydrofuran, wherein the molar ratio of phenol to hexachlorocyclotriphosphazene was 4.2:1, and the reaction was carried out at room temperature for 12 hours, and compound N was obtained after purification. 3 P 3 (OC 6 H 5 ) 4 Cl 2 . Wherein each mole of phenol is dissolved in 100 ml of tetrahydrofuran, and each mole of hexachloroc...
example 2
[0060] When the structure of R is C 6 H 4 CH 2 C 6 H 4 , the structural formula of the synthesized cyclic linear phosphazene epoxy resin CLPN-EP2 is:
[0061]
[0062] n is an integer between 0-10.
[0063] The synthetic embodiment of CLPN-EP2 is as follows:
[0064] In step 1, under the protection of argon atmosphere, phenol is dissolved in tetrahydrofuran and reacted with sodium hydride at room temperature for 2 hours to obtain a corresponding reaction mixture solution. The reaction mixture solution was added dropwise to a solution of hexachlorocyclotriphosphazene dissolved in tetrahydrofuran, wherein the molar ratio of phenol to hexachlorocyclotriphosphazene was 4.3:1, and the reaction was carried out at room temperature for 12 hours to obtain compound N after purification. 3 P 3 (OC 6 H 5 ) 4 Cl 2 . Wherein each mole of phenol is dissolved in 100 ml of tetrahydrofuran, and each mole of hexachlorocyclotriphosphazene is dissolved in 120 ml of tetrahydrofuran...
example 3
[0071] When the structure of R is C 6 H 4 SO 2 C 6 H 4, the structural formula of the synthesized cyclic linear phosphazene epoxy resin CLPN-EP3 is:
[0072]
[0073] n is an integer of 0-10.
[0074] The synthetic embodiment of CLPN-EP3 is as follows:
[0075] In step 1, under the protection of argon atmosphere, phenol is dissolved in tetrahydrofuran and reacted with sodium hydride at room temperature for 2 hours to obtain a corresponding reaction mixture solution. The reaction mixture solution was added dropwise to a solution of hexachlorocyclotriphosphazene dissolved in tetrahydrofuran, wherein the molar ratio of phenol to hexachlorocyclotriphosphazene was 4.1:1, and the reaction was carried out at room temperature for 13 hours to obtain compound N after purification. 3 P 3 (OC 6 H 5 ) 4 Cl 2 . Wherein each mole of phenol is dissolved in 100 ml of tetrahydrofuran, and each mole of hexachlorocyclotriphosphazene is dissolved in 120 ml of tetrahydrofuran.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com