Medicine T-VISA-PEA15 capable of effectively and specifically killing breast cancer cells
A T-VISA-PEA15, breast cancer cell technology, applied in the field of medicine and biology, can solve the problems of no tissue specificity of the promoter, low promoter activity, and large toxic and side effects, and achieve no liver and kidney toxicity and high gene expression. , the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
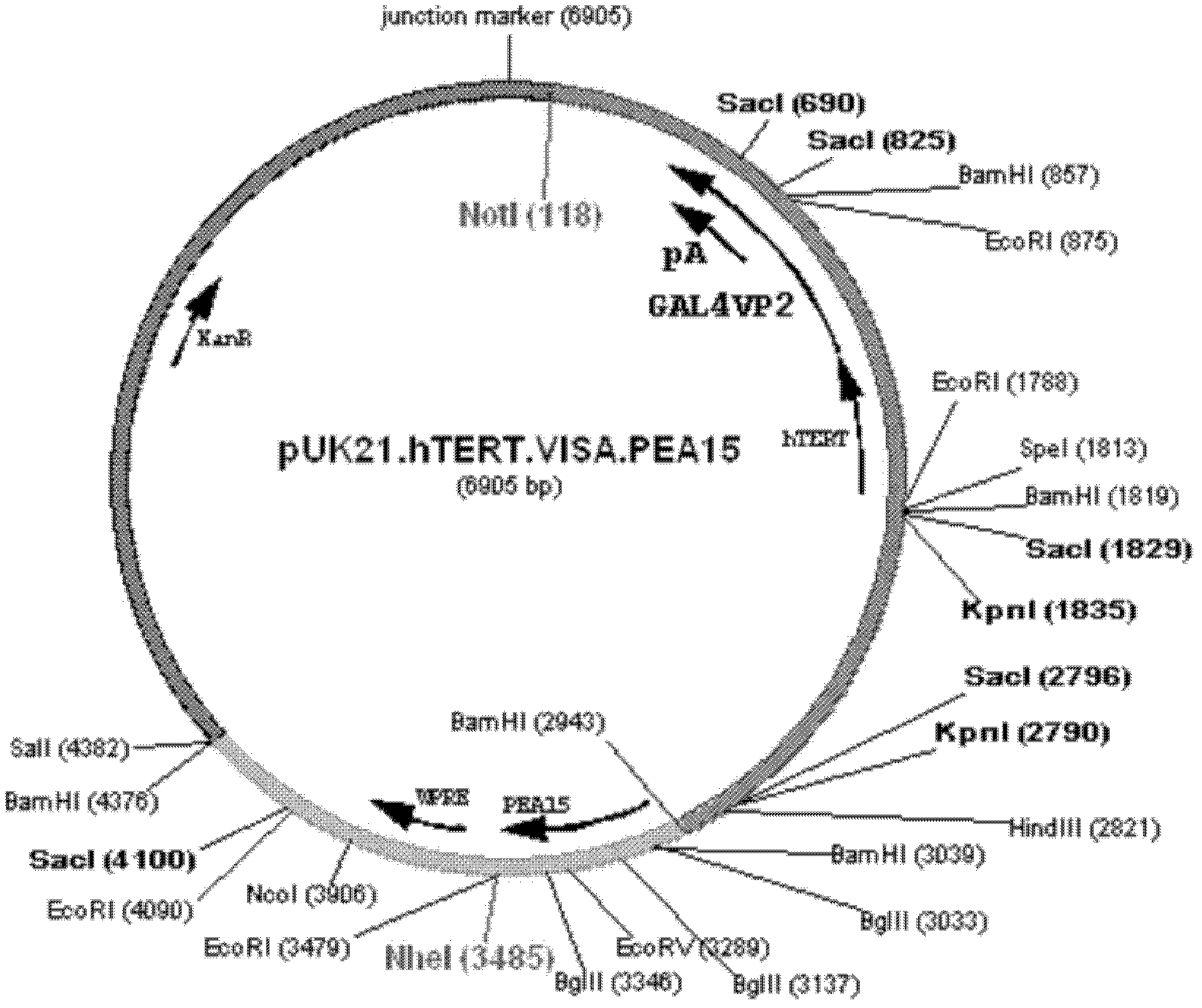
[0018] Example 1: Construction of T-VISA-PEA15 therapeutic vector
[0019] (1) pCRII-TOPO-hTERT plasmid cloning
[0020] 1. The DNA of breast cancer MCF-7 cells (purchased from ATCC, USA) was extracted by conventional methods.
[0021] 2. Using the DNA as a template, design PCR primers for amplifying hTERT, hTERT PCR upstream (Forward) and downstream (Reverse) primers:
[0022] Forward: 5′-ata tct aga ggc ccc tcc ctc ggg tta ccc cac agc-3′(XbaI)
[0023] Reverse: 5′-ata gat ctt atg cgg ccg ccc acg tgc gca gca gga cgc agc gc-3′.
[0024] 3. Using breast cancer MCF-7 cell DNA as a template, hTERT PCR primers (Forward: 5′-ata tct aga ggc ccc tcc ctc ggg tta ccc cac agc-3′; Reverse: 5′-ata gat ctt atg cgg ccg ccc acg tgc gca gca gga cgc agc gc-3'), Pfu DNA polymerase, dNTP and PCR reaction solution, amplified to obtain hTERT promoter (-416 to +1) product, its sequence is shown in SEQ ID NO.2. Its PCR reaction system: 10×PCR buffer 5 μl, upstream (Forward) and downstream (Rever...
Embodiment 2
[0080] Embodiment 2: the preparation of liposome:
[0081] Lipids were taken out from the refrigerator (DOTAP was stored at -20°C, cholesterol was stored at -4°C) and returned to room temperature. Two rotary evaporators were heated in a water bath to 30°C and 50°C respectively. Weigh 68.75mg of cholesterol and put it into a 1000ml round bottom flask. Into a round bottom flask was added 100 mg DOTAP and 25 mg Chloroform. Swirl the flask to mix well. Rotate the round-bottom flask in a water bath at 30°C for 2 minutes to make it evenly mixed and form a film on the wall of the flask. Turn on the vacuum aspirator, 30min at 30°C. Add 8.9ml of preheated 5% glucose solution to dissolve the dried film, and rotate rapidly at 105 rpm for 45min at 50°C. Then lower the temperature to 35 °C and rotate for 10 min. Seal the flask with plastic wrap (or paraffin), and store it overnight at room temperature, away from light. Measure the volume, add double distilled water to 8.9ml. The fl...
Embodiment 3
[0082] Embodiment 3: the preparation of T-VISA-PEA15 liposome
[0083] T-VISA-PEA15 treatment carrier is dissolved in 5% glucose solution, makes its final concentration 1ug / ul, simultaneously the liposome of the embodiment 2 of storage concentration (20mM) is diluted to working concentration (8mM) with 5% glucose solution . Add 1μg DNA / ul T-VISA-PEA15 therapeutic carrier slowly into 8mM liposomes (therapeutic carrier: liposome = 20ug: 50ul), let stand at room temperature for 20 minutes, react to form T-VISA-PEA15 lipid plastid.
[0084] Then test, biological characteristic detection and quality control such as endotoxin, aseptic packaging. Finished product testing, packaging, storage at 4-8°C; put at room temperature for 20 minutes before use, can be used directly for in vitro research, can also be used for in vivo research, and can also be used after dilution with 5% glucose solution.
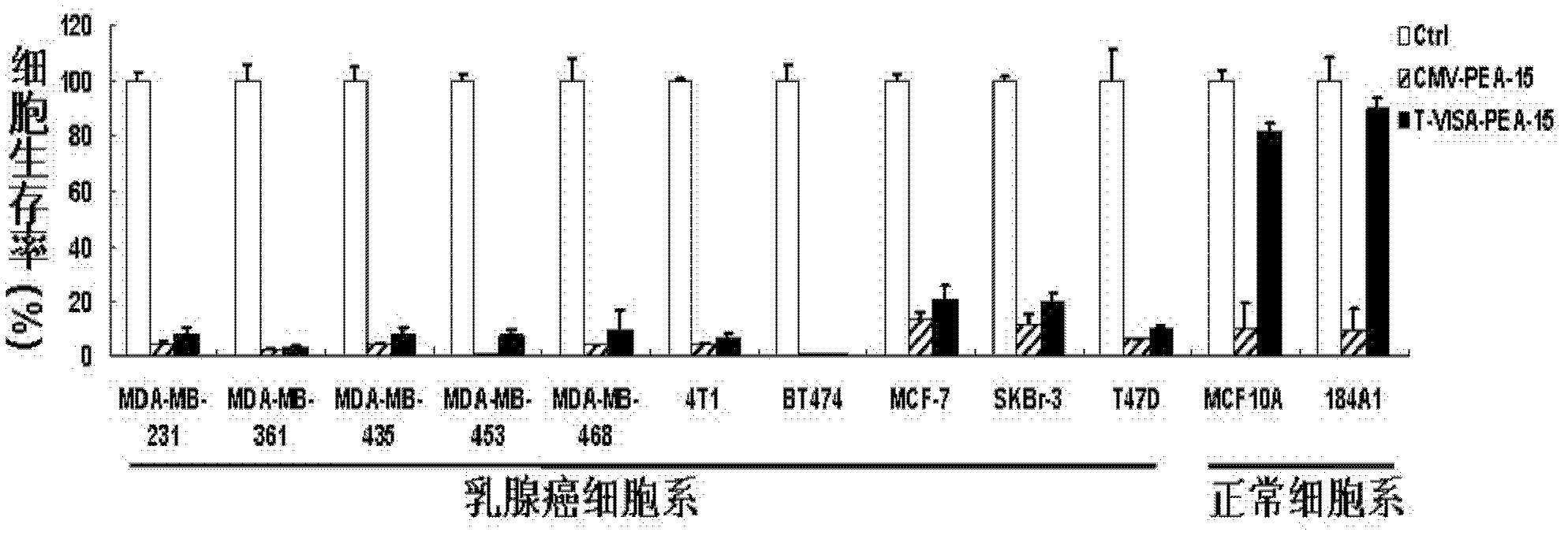
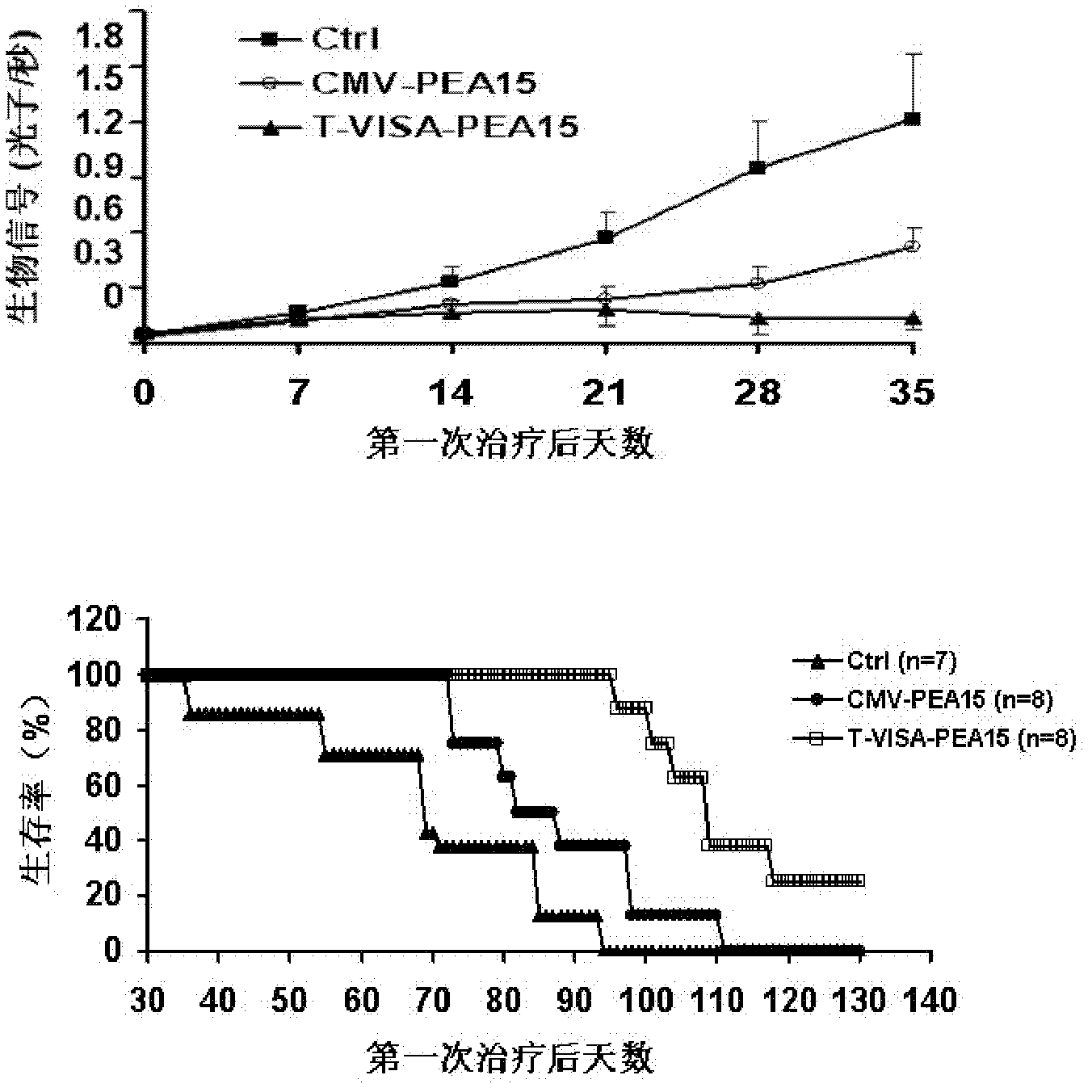
[0085] 2. Effect experiment:
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com