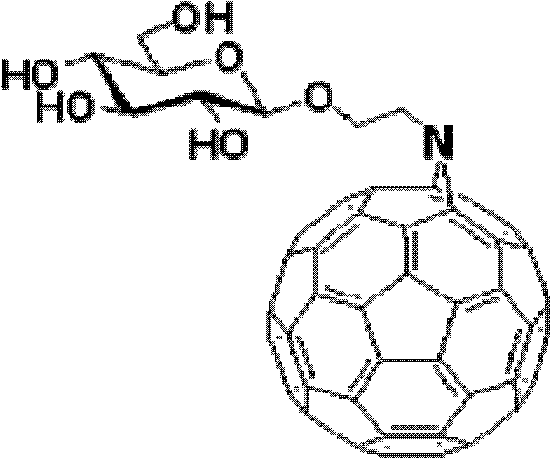
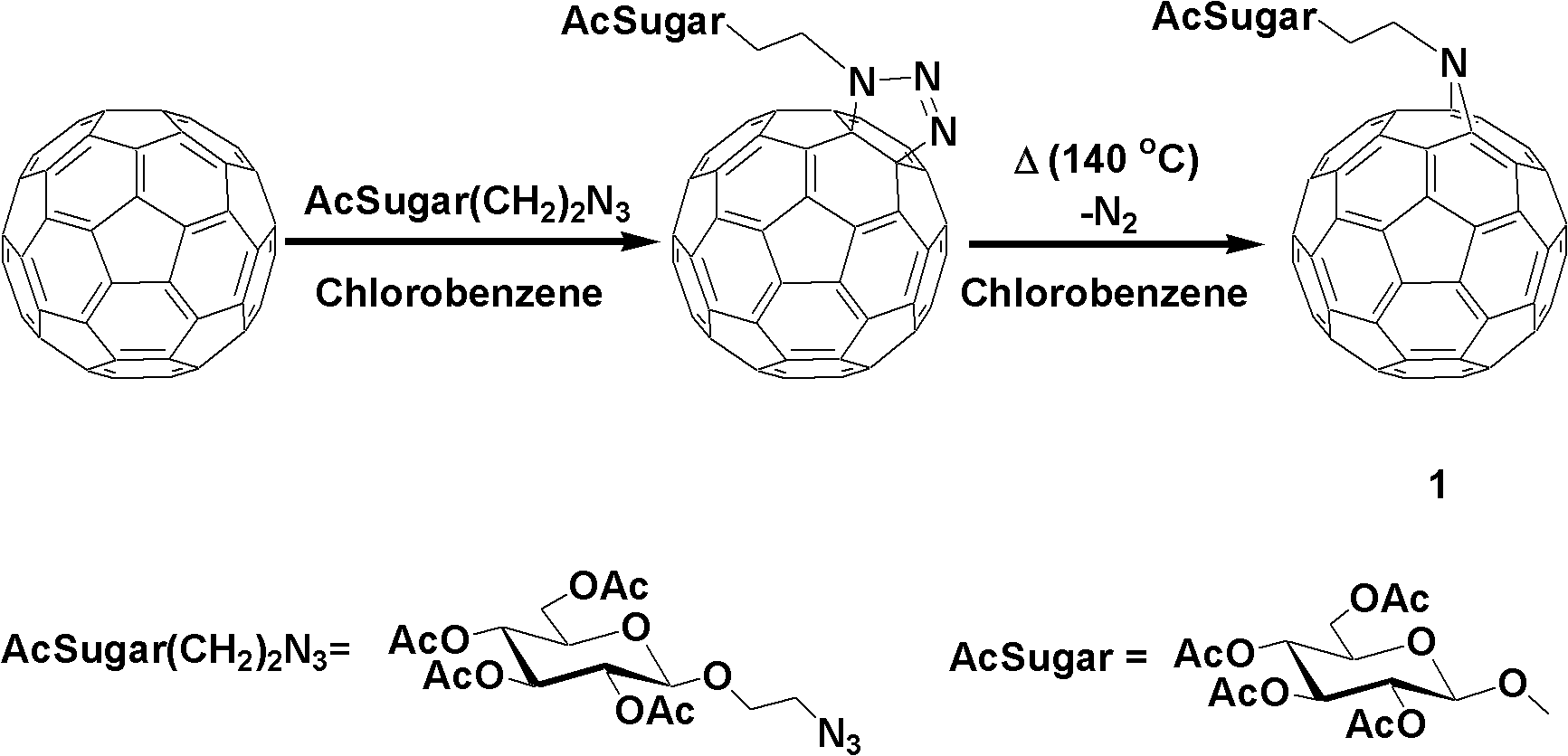
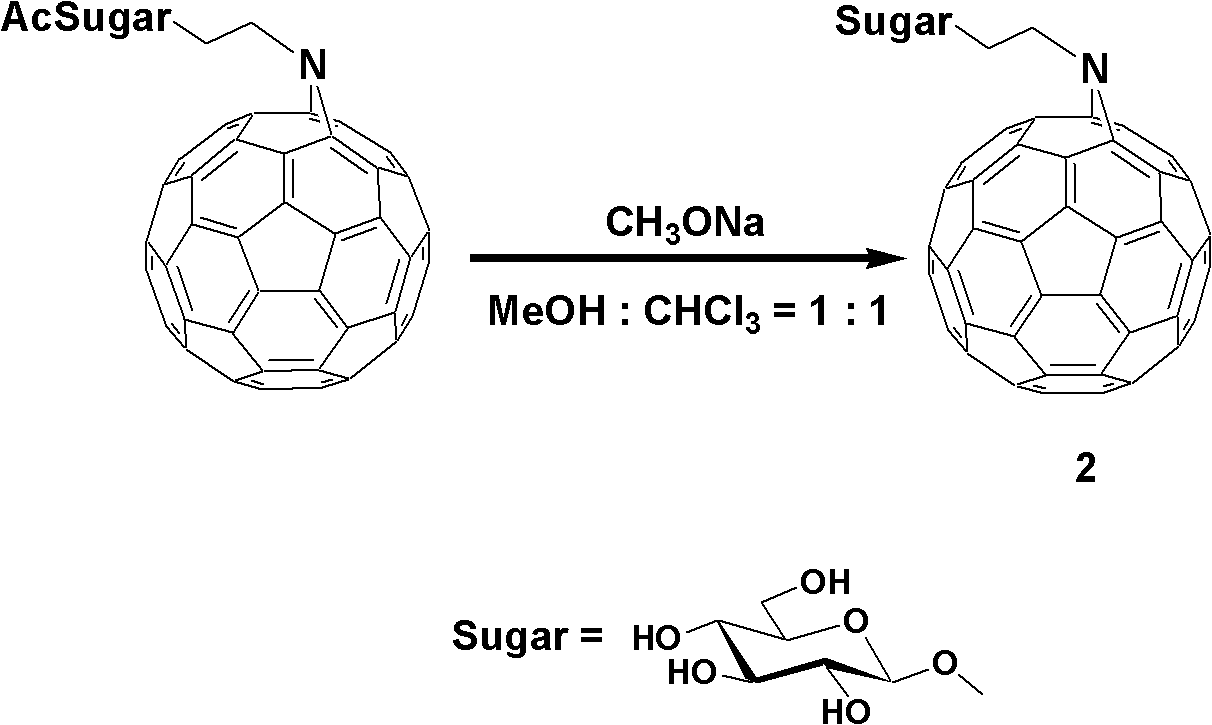
Fullerene polysaccharide derivative and its preparation method
A technology of fullerene polysaccharides and fullerene sugars, which is applied in the field of novel fullerene polysaccharide derivatives and preparation, and the preparation of organic polymer materials, and can solve the problems affecting the promotion of fullerene-derived drugs and poor biocompatibility. problem, to achieve the effect of improving medical level and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The following examples describe the present invention in more detail:
[0020] Synthesis of 1,2,3,4,6-penta-O-acetyl-β-D-glucose
[0021] Add 350ml of anhydrous acetic acid to anhydrous sodium acetate (25.2g, 0.307mol), heat to 140°C, and slowly add 50.0g of D-glucose while the system is boiling. After the addition is complete, continue heating to boiling until the reaction is complete, and place it at room temperature to cool. The reaction solution was poured onto 1 liter of ice cubes, and a brown precipitate was formed after stirring for 4 hours. After suction filtration, rinse with water until there is no smell of acetic acid, and dry under reduced pressure. The obtained substance was added to 400 mL of ethanol, and stirred while heating to dissolve it. After adding activated carbon and stirring thoroughly, suction filter while hot to remove activated carbon. The obtained solution was continued to be heated, the solvent was evaporated to a normal amount, cooled n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com