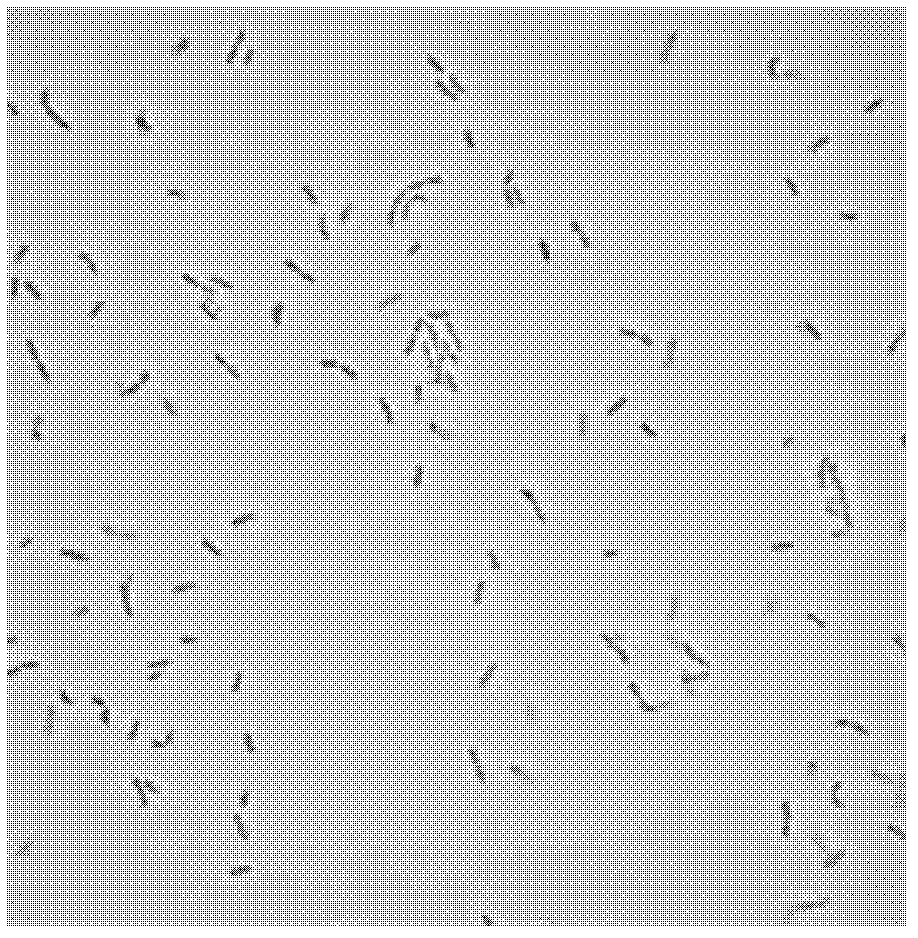Novel lactic acid bacterium strains and application thereof to adjustment of immune reaction
A technology of lactic acid strains and strains, applied in the directions of application, medical preparations containing active ingredients, bacteria, etc., can solve the problem that lactic acid strains have not been found yet, and achieve the effect of inhibiting Th2 immune response and reducing bacterial infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Isolation and cultivation of bacterial strain
[0050] Stool specimens were collected from healthy infants in Taiwan, and cultured at 37°C for 48 to 72 hours on Rogosa plate medium, which is a selective medium for Lactobacillus, to obtain colonies of suspected strains of Lactobacillus. Get the culture and spread it on the MRS plate, culture it anaerobically at 37°C for 48 to 72 hours, select a single colony growing on the plate for further purification, and carry out strain identification according to the description in Example 2 to obtain isolated strains MP137 and MP108.
[0051] Inoculate the isolated strain on the MRS plate, and after anaerobic culture for 48 to 72 hours, pick a single colony and inoculate it into fresh MRS culture medium. After the strain grows well (the turbidity can be judged by naked eyes), then take 1% bacterial liquid Transfer to another fresh MRS culture medium, incubate at a suitable temperature for 18 to 24 hours, repeat this...
Embodiment 2
[0052] Embodiment 2: bacterial strain identification
[0053] 2.1 Preliminary analysis
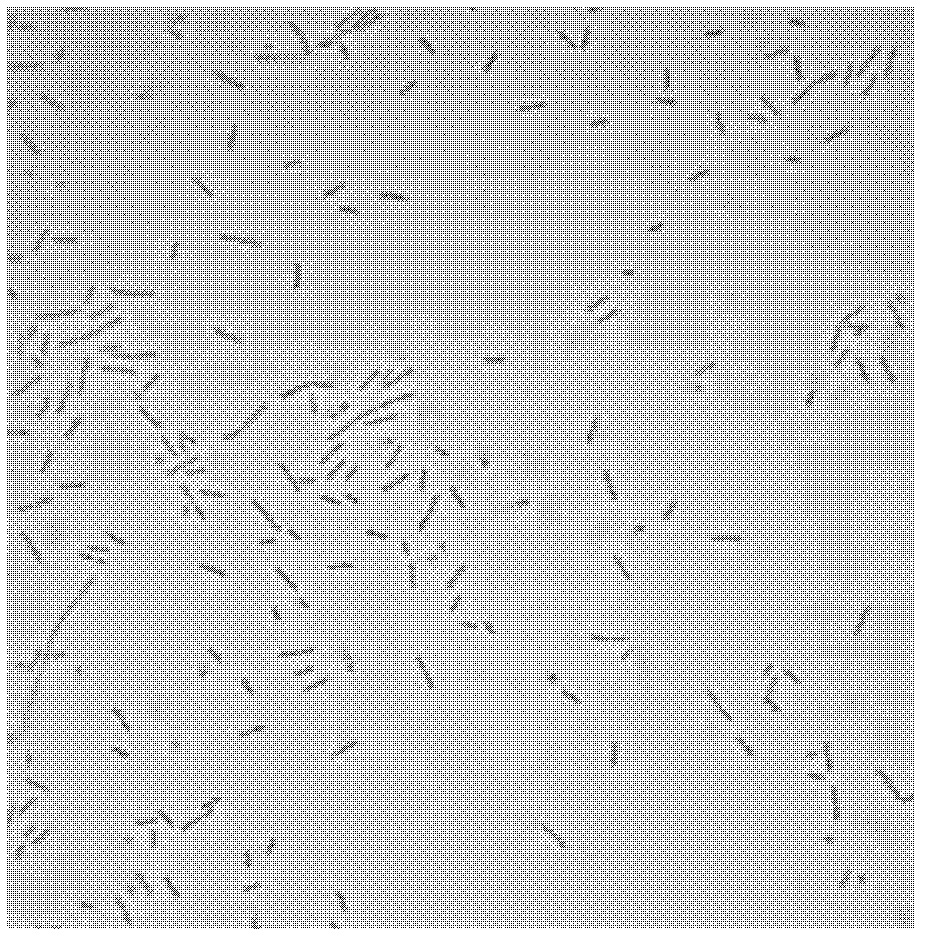
[0054] Carry out preliminary analysis according to the standard method, the result shows that isolated bacterial strain MP137 of the present invention and MP108 are Gram-positive bacilli ( figure 1 and 2 ), and does not have catalase, oxidase and motility, does not produce endospores, and can grow in both aerobic and anaerobic environments.
[0055] 2. 216SrDNA PCR analysis
[0056] 16S rDNA PCR analysis was carried out for the isolated strains MP137 and MP108 of the present invention. Use the commercial kit (AxyPrep Bacterial Genomic DNA Miniprep Kit, Anxygen Bioscience) to extract the genomic DNA of the strain as a template, and add forward and reverse primers (16S-F: GGAGTTTGATCCTGGCTCAG (SEQ ID NO: 1); and 16S-R2 : AAGGAGGTGAT CCAGCCGCA (SEQ ID NO: 2)), DNA polymerase, buffer, dNTPs and other reagents, the contents are as follows:
[0057]
[0058] 16S rDNAPCR reaction conditio...
Embodiment 3
[0075] Example 3: Analysis of Immunomodulation
[0076] 3.1 Cell experiments
[0077] 3.1.1 Preparation of heat-killed bacteria liquid
[0078] Take the cultured bacteria liquid of the aforementioned isolates MP108 and MP137, put it into a centrifuge tube, place it in a water bath and boil it for 30 minutes to prepare a heat-killed bacterial body liquid. Bacterial fluid concentration is 1x10 10 cfu / ml, stored at 4°C for subsequent experiments.
[0079] 3.1.2 Purification of CD3+ T cells
[0080] Collect about 100ml of venous blood from a healthy person, take 25ml of diluted blood and slowly add it to a centrifuge tube containing 20ml of Ficoll-Hypaque, centrifuge at 400xg for 40 minutes, and use the difference in density to separate the peripheral blood into a single nucleus. Spheroid cells were detached, washed again with phosphate buffer, and the number of cells was counted. Add the peripheral blood mononuclear leukocytes (PBMCs) isolated from human peripheral blood int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com