Composition for improving, preventing or treating skin diseases comprising induced pluripotent stem cell-derived mesenchymal stem cell and exosome derived therefrom
A technology of pluripotent stem cells and skin diseases, applied in the field of compositions for improving, preventing or treating skin diseases comprising mesenchymal hepatocytes derived from induced pluripotent stem cells and exosomes derived therefrom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
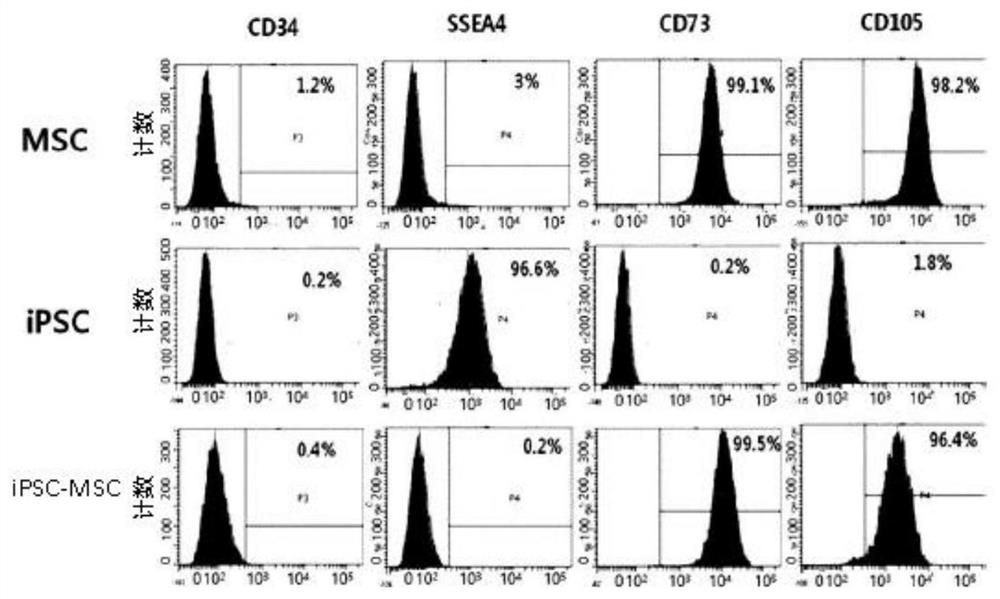
[0106] Example 1: Preparation and analysis of mesenchymal stem cells derived from induced pluripotent stem cells
[0107] In a state that does not use supporting cells (cultivated auxiliary cells), it contains knock-out xeno-free serum replacement, glutamine, non-essential amino acids, β-mercaptoethanol, antibiotics, and basic ingredients. Induced pluripotent stem cells (fibroblasts and peripheral blood mononuclear cells cultured in Asan Hospital Stem Cell Center, Seoul) were cultured in DMEM / F-12 medium with bFGF (basic fibroblast growth factor). (Peripheral Blood Mononuclear Cell, PBMC, induced pluripotent stem cell line of mesenchymal stem cells) was attached to a culture dish (dish) coated with vitronectin in advance, and added with 10% fetal bovine serum ( v / v), 5ng / ml basic fibroblast growth factor, 0.1mM minimum essential medium non-essential amino acids (MEMNEAA, Minimum Essential Media Non-Essential Amino Acids), β-mercaptoethanol (β-mercaptoethanol) ( 1×), 100 unit / ...
Embodiment 2
[0118] Example 2: Isolation and confirmation of exosomes derived from mesenchymal cells differentiated from induced pluripotent stem cells
[0119] The iMSCs whose characteristics of mesenchymal stem cells were confirmed were additionally cultured in a medium supplemented with 10% exosome-depleted bovine serum (Exosome-depleted FBS). After culturing for 72 hours, the culture medium of recovered iMSCs was centrifuged at 300×g for 10 minutes to remove remaining cells and cell residues. The supernatant was filtered with a 0.22 μm filter, and then centrifuged at 10,000×g and 4° C. for 70 minutes using a high speed centrifuge. Use an ultracentrifuge (ultracentrifuge) at 100,000×g, 4°C to centrifuge the centrifuged supernatant for 90 minutes, remove the supernatant, and dilute the remaining supernatant with phosphate buffered saline (PBS, phosphate buffered saline). Exosomes are used.
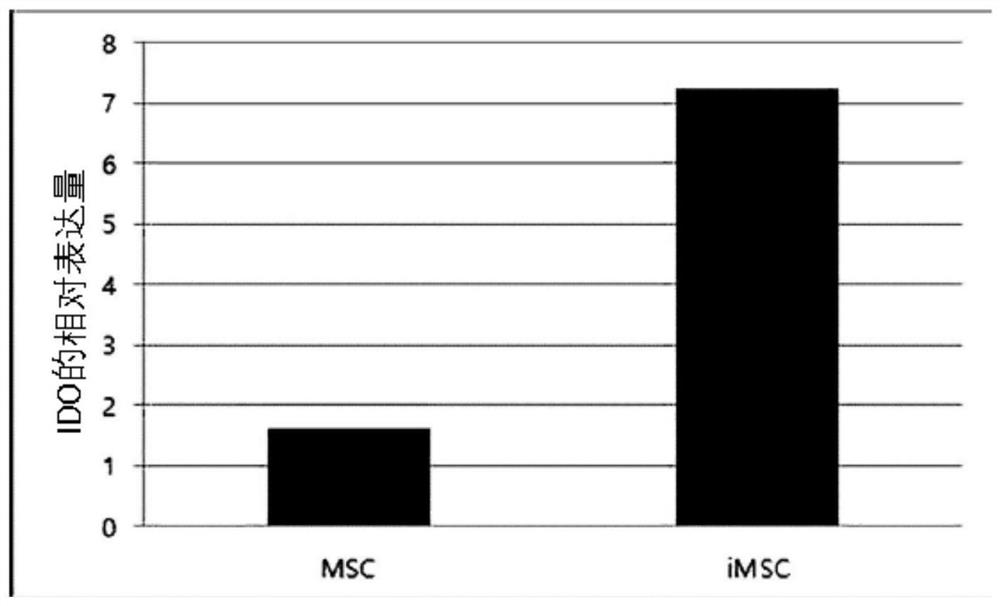
[0120] The number and size distribution of exosomes isolated from MSCs and iMSCs were confirmed...
Embodiment 3
[0122] Example 3: Preparation and administration of mice induced with allergic diseases
[0123] The animals used in the experiment were purchased 8-week-old female BALB / c mice (Orient, Korea), which were acclimatized for one week and then used in the experiment at 9-week-old. To induce atopic dermatitis, depilation was performed on the back of BALB / c mice up to the upper part of the back with the largest epilator. 1×1cm on the depilated back at intervals of 24 hours 2 40 μg of Aspergillus fumigatus (Af, Aspergillus fumigatus ) extract was applied to the area of ??for 5 days. After a rest period of 2 weeks, the application was repeated 5 times at intervals of 24 hours from the 19th day, thereby completing the preparation of the animal model of allergic dermatitis.
[0124] After the animal model of allergic dermatitis was prepared, MSC, iMSC, MSC-Exo (exosomes derived from mesenchymal stem cells) or iMSC-Exo (mesenchymal stem cells derived from induced pluripotent stem cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com