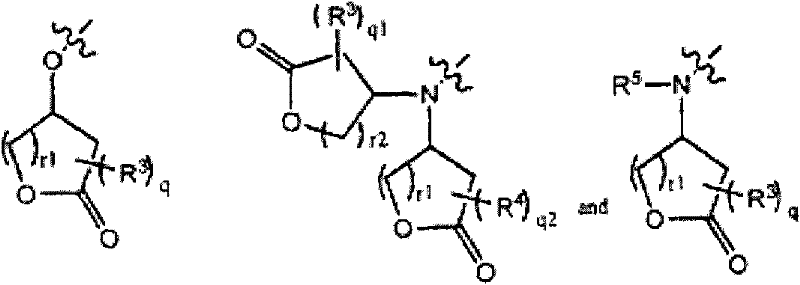
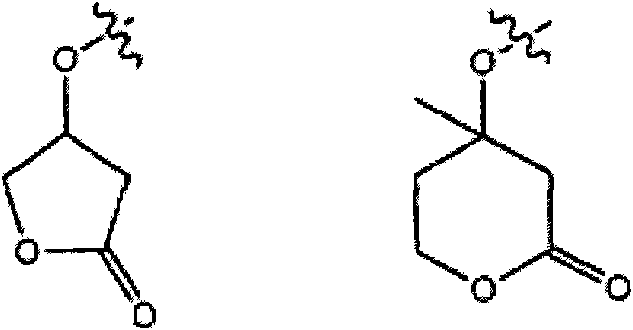Base reactive photoacid generators and photoresists comprising same
A photoacid generator and photoresist technology, applied in the field of photoresist compositions, can solve the problems of no beneficial effect of photoresist lithography effect, slow alkali-promoted cracking, etc., so as to improve exposure Tolerance, Defect Reduction, Effect of Low PAG Acid Diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] The photoacid generator TPS was prepared by Equation 1 and the multi-step synthesis outlined below
[0093] NBHFA-TFPS. The detailed synthesis method is shown below.
[0094] Reaction 1
[0095]
[0096] To a solution of 4-bromo-3,3,4,4-tetrafluorobutyric acid (1.26 g, 102.7 mmol) in 150 mL of tetrahydrofuran was added carbonyldiimidazole (CDI, 16.7 g, 103.0 mmol), and the mixture was stirred at room temperature 2 Hour. The reaction mixture was heated to 70°C, then compound 2 (30 g, 102.6 mmol) was added to the reaction at 70°C under nitrogen atmosphere for 16 hours. The solvent was removed under reduced pressure and the resulting oily residue was dissolved in 200 mL of dichloromethane. The latter solution was washed twice with 200 mL of 1N HCl, once with 200 mL of water, MgSO 4 Drying and removal of the solvent under reduced pressure afforded compound 3 as a colorless oil.
[0097] In the second step, compound 3 (45 g, 85.35 mmol) was dissolved in 200 mL of ac...
Embodiment 2
[0099] Example 2: Lithographic Evaluation
[0100] The photoacid generator TPS NBHFA-TFPS of Example 1 was evaluated by photolithography with respect to conventional PAG (triphenylsulfonium perfluorobutanesulfonate). Photoresists were prepared using the components and parts described below.
[0101] The photoresist polymer (A1 ) used in the lithography evaluation (below) was prepared according to the method described below using the monomers M1-M5 described below.
[0102]
[0103] 1-ethylcyclopentyl methacrylate (ECPMA, M1; 20mmol), 1-isopropyl-adamantyl methacrylate (IAM, M2; 20mmol), 2-oxo-tetrahydromethacrylate -furan-3-yl ester (α-GBLMA, M3; 30mmol), 3-oxo-4,10-dioxo-tricyclo[5.2.1.02,6]dec-8(or 9)-ylmethacrylic acid Esters (ODOMA, M4; 20 mmol) and 3-hydroxy-adamantyl methacrylate (HAMA, M5; 10 mmol) were dissolved in 30 g of tetrahydrofuran (THF) for degassing by bubbling with nitrogen, and injected into the formulation. An additional 10 g of degassed THF was added...
Embodiment 3
[0112] Example 3: Synthesis of (4-((2,2-difluoro-3-(methacryloyloxy) (acryloyloxy)) propionyl) oxo)phenyl)-diphenylsulfonium perfluorobutanesulfonate
[0113]
[0114] 2,2-Difluoro-3-(methacryloxy)propionic acid: Add 12.6 g (100 mmol) of 2,2-bis to a mixture of 250 mL of dichloromethane and 10.1 g (100 mmol) of triethylamine Fluoro-3-hydroxypropionic acid, and the resulting mixture was placed in an ice bath. 10.5 g (100 mmol) of methacryloyl chloride was slowly added to the sesame cake, and the reaction was stirred overnight. The resulting mixture was then treated with 200 mL of 1% NaHCO 3 The solution was washed, followed by removal of solvent. The product was recrystallized from methanol to give 2,2-difluoro-3-(methacryloyloxy)propionic acid in expected good yield, which was directly used in the following synthesis without further purification.
[0115] (4-((2,2-difluoro-3-(methacryloyloxy)propionyl)oxy)phenyl)-diphenylsulfonium perfluorobutanesulfonate: to 5g (25.7mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com