Preparation method of functionalized aryl acetate compound
A technology of aryl acetate and functional grouping, which is applied in the field of preparation of aryl acetate compounds, can solve problems such as narrow scope of application, and achieve the effects of good compatibility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] In the preparation method provided by the present invention, the above-mentioned reactant is preferably placed in the following organic solvent for reaction: benzene, toluene, xylene, 1,3,5-trimethylbenzene, dimethyl sulfoxide, N,N-dimethyl Formamide, N,N-dimethylacetamide, N-methylpyrrolidone, diethylene glycol dimethyl ether, diethylene glycol diethyl ether, triethylene glycol dimethyl ether, or dipropylene glycol diethyl ether. The inert gas can be a gas well known to those skilled in the art, such as argon or nitrogen atmosphere. The reaction temperature is preferably 120°C to 140°C, and the reaction is completed after 10 to 30 hours of reaction time, and then the product is purified as follows:
[0058] After the reaction system mixture that has been reacted is filtered, the filtrate is chromatographed using fast silica gel column chromatography, and the reaction solvent is first eluted with petroleum ether, and then the mixed solvent eluent of petroleum ether and ...
Embodiment 1
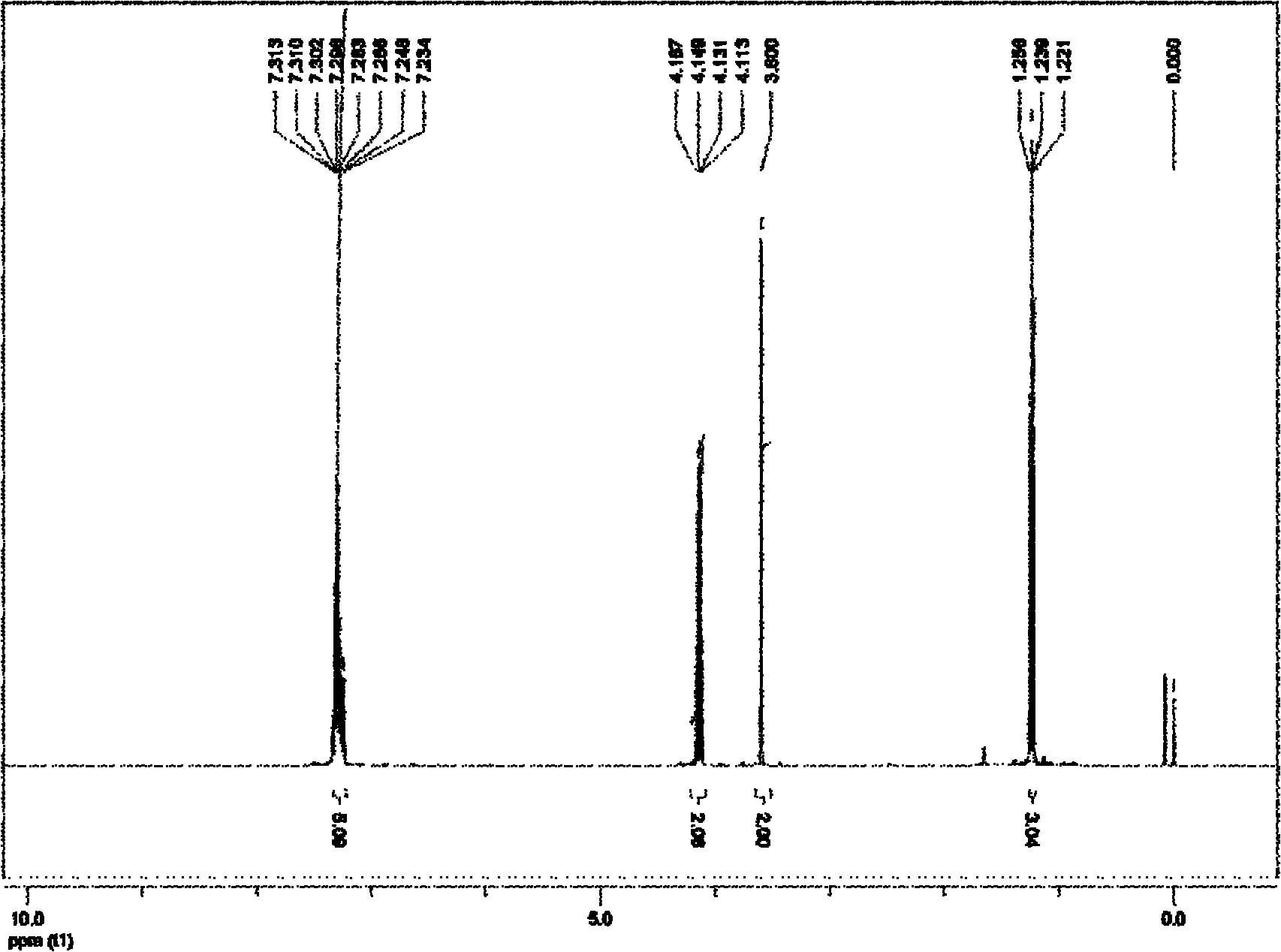
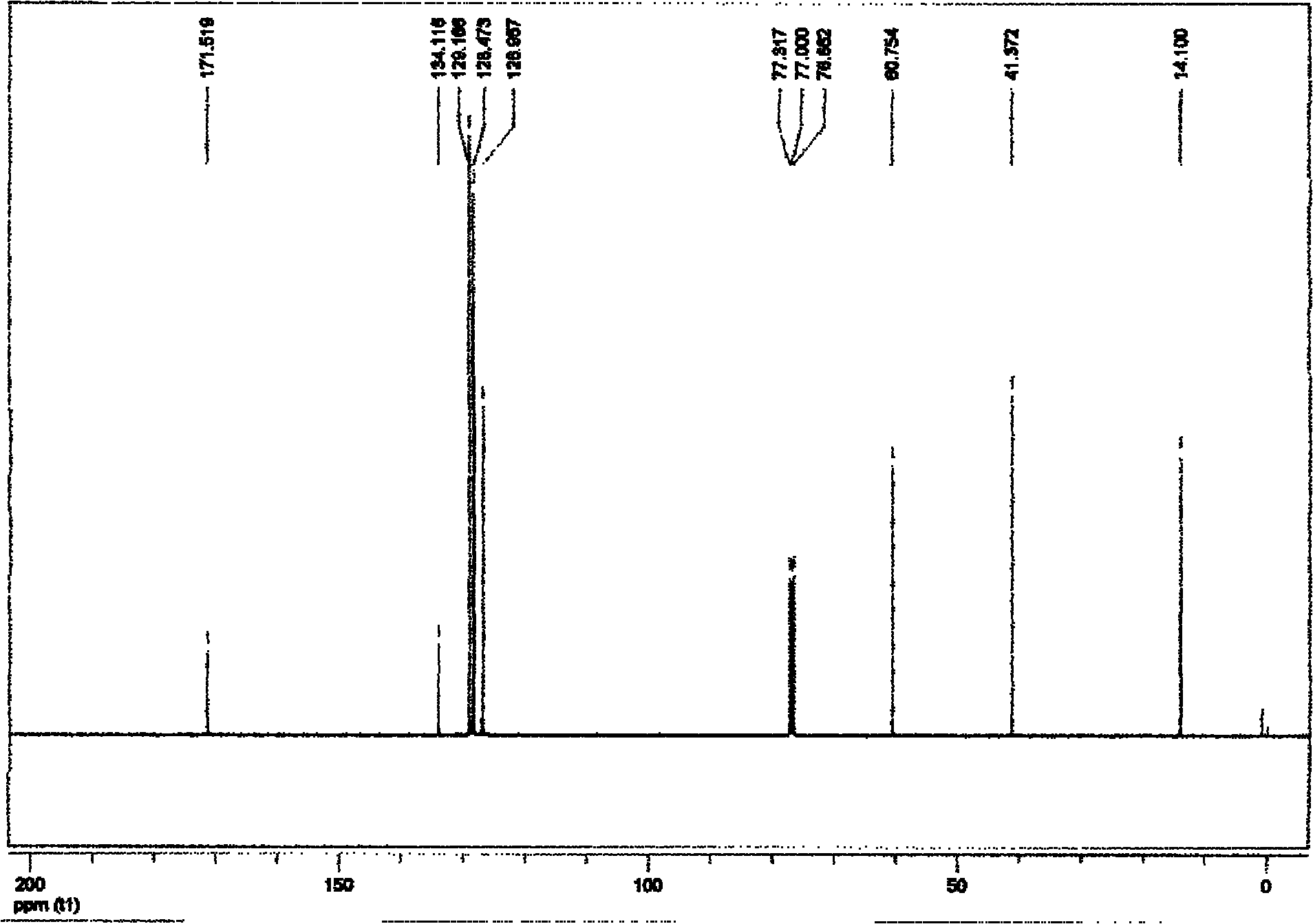
[0061] Embodiment 1 prepares ethyl phenylacetate
[0062] 1. Add 0.75 mmol of monoethyl malonate potassium salt, 0.01 mmol of allyl palladium chloride and 0.03 mmol of 2-bicyclohexylphosphine-2', 6'-diisopropoxy in a vacuum reactor Base biphenyl, vacuumize, connect high-purity argon, replace three times, add 0.5mmol of chlorobenzene and organic solvent trimethylbenzene under the protection of argon flow, the addition of organic solvent adds 2 In milliliters of solvent, the mixture in the reactor was heated to 120° C., and the reaction was terminated after heating and stirring for 10 hours. The reaction of this step is as follows:
[0063]
[0064] 2. Purify the reaction system mixture after the reaction with fast silica gel column chromatography, first use 50mL of petroleum ether to elute the solvent trimethylbenzene, and then use petroleum ether and ethyl acetate as an eluent with a volume ratio of 50:1. The product ethyl phenylacetate was obtained in 83% yield.
[0065...
Embodiment 2
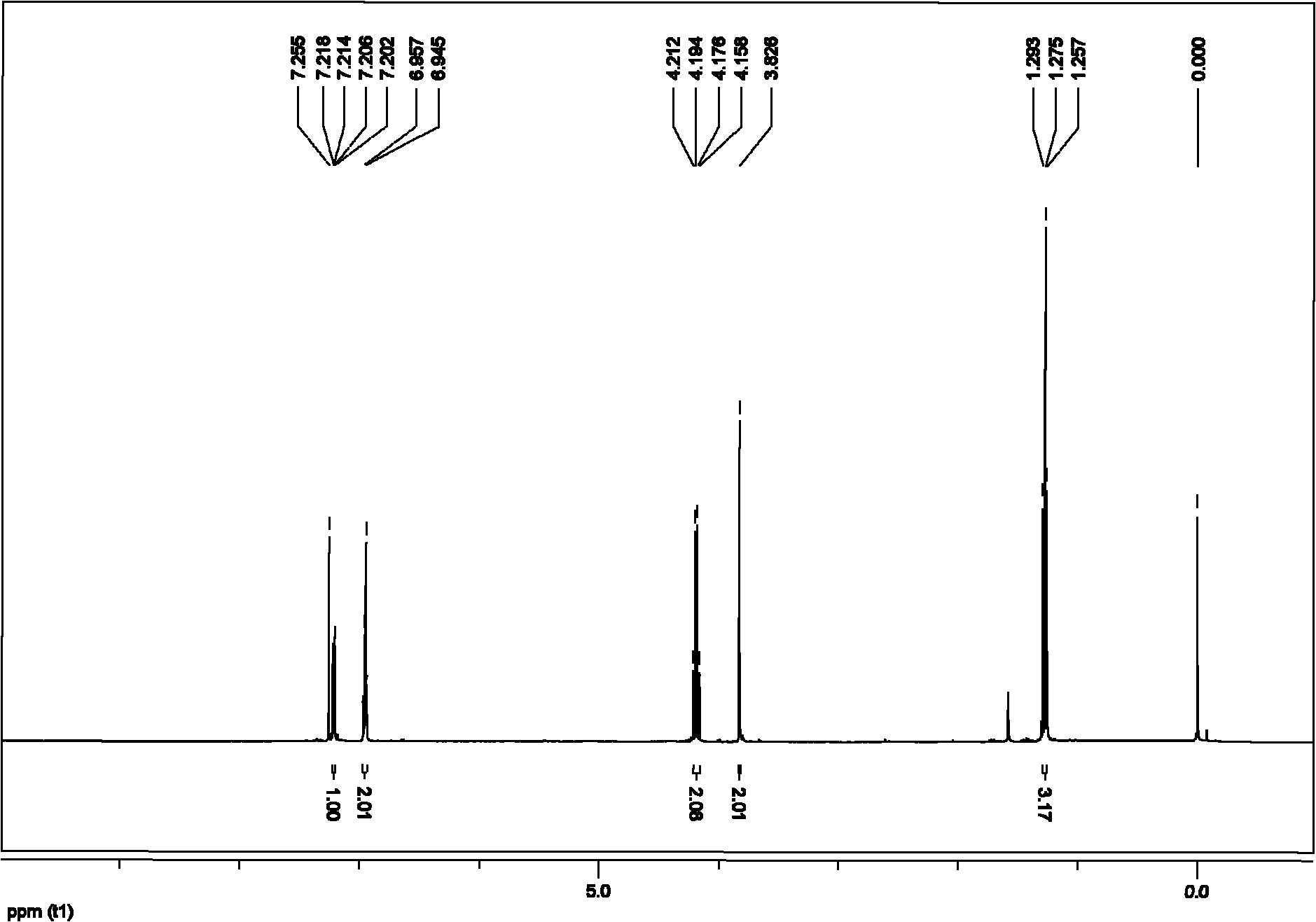
[0066] Embodiment 2 prepares 2-(2-thienyl) ethyl acetate
[0067] 1. Add 0.75mmol of monoethyl malonate potassium salt, 0.01mmol of allyl palladium chloride (Pd2(p-allyl)2Cl2) and 0.03mmol of 2-bicyclohexylphosphine-2 in the vacuum reactor ', 6'-diisopropoxybiphenyl, vacuumize, pass through high-purity argon, replace three times, add 0.5mmol of pro-2-chlorothiophene and organic solvent trimethylbenzene under the protection of argon flow, and organic solvent The addition amount is based on the addition of 2 milliliters of solvent per millimole of the electrophilic substrate, and the mixture in the reactor is heated to 140° C., and the reaction is terminated after heating and stirring for 20 hours. The reaction of this step is as follows:
[0068]
[0069] 2. Purify the reaction system mixture after the reaction with fast silica gel column chromatography, first use 50mL of petroleum ether to elute the solvent trimethylbenzene, and then use petroleum ether and ethyl acetate a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com