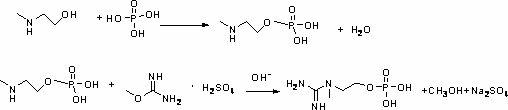Method for synthesizing creatinol-o-phosphate
A technology of inositol phosphate and synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of serious environmental pollution, high equipment and operation requirements, and achieve the effects of no environmental pollution, simple and easy operation, and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] A kind of synthetic method of inositol phosphate, comprises the steps:
[0018] (1) Using N-methyl-2-aminoethanol as raw material, reacting with phosphoric acid to prepare N-methyl-2-aminoethanol phosphate;
[0019] (2) React N-methyl-2-aminoethanol phosphate obtained in step (1) with O-methylisourea sulfate under alkaline conditions to prepare inositol phosphate.
[0020] Wherein, in step (1), the molar ratio of N-methyl-2-aminoethanol to phosphoric acid is 1:1-1.1, and the reaction conditions are: reaction temperature 0-180°C, reaction time 2 ~6h, the preferred reaction conditions are: reaction temperature 40~150°C, reaction time 3~5h; in step (2), the N-methyl-2-aminoethanol phosphate and O-methylisourea sulfuric acid The molar ratio of the salt is 1:1-1.1, and the reaction conditions are: reaction temperature 0-60°C, reaction time 1-4h, preferred reaction conditions are: reaction temperature 10-50°C, reaction time 2-3h.
[0021] The chemical equation of this react...
Embodiment 1
[0024] Add 75.8g of methylethanolamine and 100ml of water into a 1000ml four-neck flask, add 99.1g of phosphoric acid dropwise at a temperature of 30°C, raise the temperature to 160°C, concentrate to dryness under reduced pressure, cool down to 90°C, add 200ml of water, and control the temperature to 30°C ℃, add 186g of O-methylisourea sulfate dropwise, after the dropwise addition, keep warm for 3h, adjust the pH of the solution to 7.0 with liquid caustic soda, cool down to 0℃, filter, wash the filter cake with 100ml of ice water, and dry to obtain 155.9g of myo-inositol phosphate, the purity is 99.12%, and the yield is 79.14%.
Embodiment 2
[0026] Add 75.8g of methylethanolamine and 100ml of water into a 1000ml four-neck flask, add 110g of phosphoric acid dropwise at a temperature of 72°C, raise the temperature to 180°C, concentrate to dryness under reduced pressure, cool down to 90°C, add 200ml of water, and control the temperature to 20°C , add 176.5g of O-methylisourea sulfate dropwise, after the dropwise addition, keep warm for 1h, adjust the pH of the solution to 7.0 with liquid caustic soda, cool down to 0°C, filter, wash the filter cake with 100ml of ice water, and dry to obtain 130.2 g inositol phosphate, the purity is 99.34%, and the yield is 66.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com