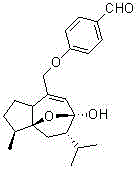
Curcumenol derivatives resisting influenza A(H1N1) virus
A technology of influenza virus and curcumol, applied in the field of medicine, can solve problems such as poor water solubility, achieve stable quality, high drug purity, and good research and development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of compound of the present invention (1)
[0042] Preparation of intermediates:
[0043] Dissolve m-CPBA (17.2g, 1mol) in dichloromethane (100ml), add curcumol (11.8g, 0.05mol) in dichloromethane (100ml) solution at 0°C, keep stirring at 0°C for 2 hours . After the reaction, the reaction liquid was extracted three times with NaOH (2 mol / l) solution, the organic phases were combined, extracted with water until neutral, and dried over anhydrous sodium sulfate. The solution was concentrated and evaporated to dryness, and the crude product was purified by silica gel column chromatography (cyclohexane:ethyl acetate=50:1~10:1) to obtain 11.95 g of white waxy solid 10,14-epoxycurcumol, yield 95% .
[0044]10,14-epoxycurcumol (10.08g, 0.04mol) was dissolved in acetonitrile (600ml), and lithium perchlorate trihydrate (6.4g, 0.04mol) and diethylamine (20ml) were added at room temperature. The resulting mixture was heated to reflux overnight. The...
Embodiment 2
[0048] Embodiment 2: the preparation of compound of the present invention (2)
[0049] Preparation of intermediate: same as the preparation method of intermediate in Example 1.
[0050] Preparation of target compound (2):
[0051] Dissolve (1S,4S,5S,7S,8R)-5,8-epoxy-9,10-ene-14-chloroguaiacol (27mg, 0.1mmol) in DMF (2ml) and add acetic acid Sodium (12.3 mg, 0.15 mmol). The resulting mixture was reacted at 70°C for 3.5 hours. The reaction solution was concentrated and evaporated to dryness, and the resulting crude product was purified by PTLC (cyclohexane:ethyl acetate=3:1, ethyl acetate eluted) to obtain colorless oily compound (1S,4S,5S,7S,8R)-5,8 - Epoxy-9,10-ene-8-hydroxy-14-guaiacol acetate (2) 21.2 mg, yield 72%. The spectral data of the compound are as follows: MS(ESI)m / z:317.0[M+Na] + ; 1 H-NMR (300MHz, CDCl 3 )δ(ppm): 1.96(1H,dd,J=8.8,16.4Hz,H-1), 1.87(1H,m,H-2a), 1.56(1H,m,H-2b), 1.87(1H, m, H-3a), 1.56(1H, m, H-3b), 1.80(1H, m, H-4), 2.19(1H, dd, J=10.7, 12.6...
Embodiment 3
[0052] Embodiment 3: the preparation of compound (3) of the present invention
[0053] Preparation of intermediate: same as the preparation method of intermediate (1S, 4S, 5S, 7S, 8R)-5,8-epoxy-9,10-ene-8,14-guaiacol in Example 1.
[0054] Preparation of target compound (3):
[0055] Dissolve (1S,4S,5S,7S,8R)-5,8-epoxy-9,10-ene-8,14-guaiarediol (25.2mg, 0.1mmol) in dry pyridine (4ml) , succinic anhydride (15mg, 0.15mmol), DMAP (2.5mg, 0.02mmol) were added. The resulting mixture was reacted at 70°C for 2 hours. The reaction solution was concentrated and evaporated to dryness, and the resulting crude product was purified by PTLC (cyclohexane:ethyl acetate=2:1, eluted with ethyl acetate) to obtain colorless oily compound (1S,4S,5S,7S,8R)-5,8 - Epoxy-9,10-ene-8-hydroxy-14-guaiacol succinate (3) 24.6 mg, yield 71%. The spectral data of the compound are as follows: MS(ESI)m / z:361.2[M+Na] + ; 1 H-NMR (300MHz, CDCl 3 )δ(ppm): 1.95(1H,dd,J=8.8,16.3Hz,H-1), 1.86(1H,m,H-2a), 1.52(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com