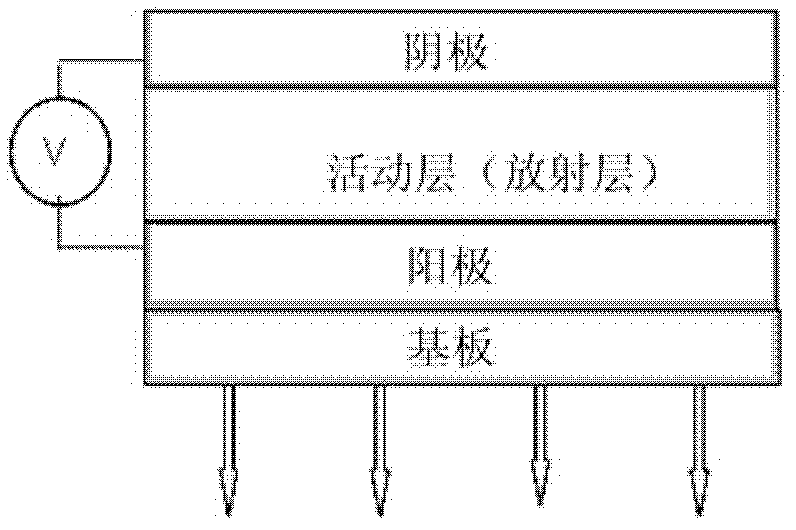
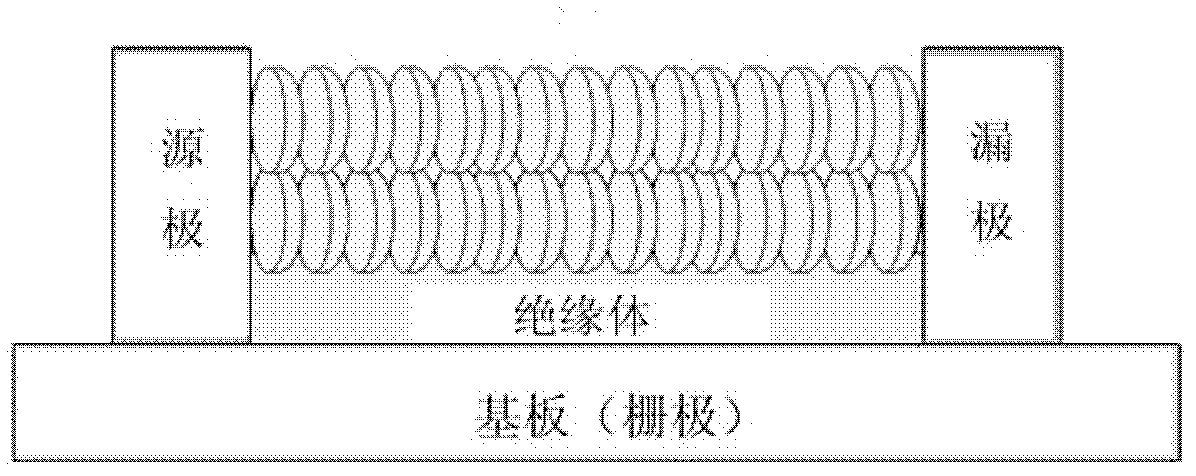
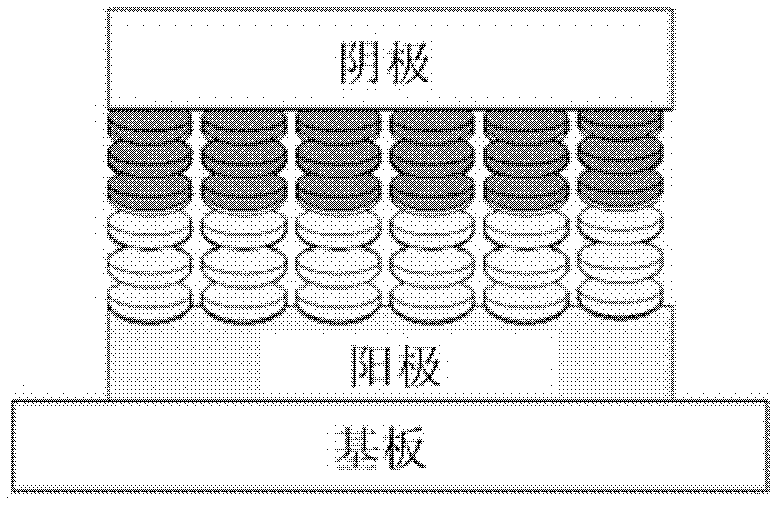
Coronene compound containing thioether in lateral chain and preparation method thereof
A technology containing thioether and benzocorone, which is applied in the fields of thioether preparation, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problems that HBC compounds have not been reported, and achieve the effect of high carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1: prepare HBC-(CH 2 ) 2 SC 10
[0113] (1) Preparation of p-bromophenethyl decyl sulfide
[0114] Put KOH aqueous solution (15ml, 6M) in the reaction flask, ventilate three times, add decyl mercaptan (10.46g, 60mmol) dropwise under nitrogen protection, after the dropwise addition, add the phase transfer catalyst tetrabutylammonium iodide (amount of catalyst, 0.8 g). Ten minutes later, 50ml of tetrahydrofuran was added, and after being uniform, p-bromophenethyl bromide (5.24g, 20mmol) was slowly added dropwise within ten minutes. The temperature was then raised to 45°C and the reaction was stirred vigorously overnight. After the reaction was completed, the reaction solution was poured into dilute hydrochloric acid (1M), extracted with dichloromethane, dried over anhydrous sodium sulfate, and evaporated to remove the solvent under reduced pressure. Column chromatography gave 6.3 g of a colorless transparent liquid with a yield of 89%.
[0115] Through nu...
Embodiment 2
[0128] (1) Preparation of p-bromophenethyl dodecyl sulfide
[0129] Using dodecylmercaptan (12.12g, 60mmol) and p-bromophenethyl bromide (5.24g, 20mmol), the method was the same as step (1) in Example 1, and the yield was 89%.
[0130] Through nuclear magnetic resonance spectrum analysis, the product obtained is p-bromophenethyl dodecyl sulfide, and the specific data are: 1 HNMR (400MHz, CDCl 3 ): δ (ppm) δ 0.86 (t, 3H, -CH 3 ), 1.29 (m, 16H, -CH 2 -), 1.33 (m, 2H, -CH 2 -), 1.52(m, 2H, -CH 2 -), 2.51(t, 2H, -CH 2 -), 2.73(t, 2H, -CH 2 -), 2.83(t, 2H, -CH 2 -), 7.07 (d, 2H, ArH), 7.40 (d, 2H, ArH). Its H NMR spectrum ( 1 HNMR) such as Figure 19 shown.
[0131] (2) 1,2-bis(dodecylthioethylphenyl)acetylene (TOLAN-(CH 2 ) 2 SC 12 )preparation
[0132] Using p-bromophenethyl dodecyl sulfide (3.85g, 10mmol), benzene (30ml), Pd(II) (dichlorobis(triphenylphosphine) palladium, 10%mol), CuI (iodide Cuprous, 10% mol), DBU (9ml), trimethylsilylacetylene (0.56g), water (0...
Embodiment 3
[0143](1) 1,2-bis(methylthioethylphenyl)acetylene (TOLAN-(CH 2 ) 2 SC 1 )preparation
[0144] Adopt p-bromophenethyl methyl sulfide (2.3g, 10mmol) (also can adopt methylmercaptan, p-bromophenethyl bromide, method is the same as the step (1) in the embodiment 1, prepare p-bromophenethyl methyl sulfide), benzene (30ml), Pd(II) (dichlorobis(triphenylphosphine)palladium, 10%mol), CuI (cuprous iodide, 10%mol), DBU (9ml), Trimethylsilylacetylene (0.56g), water (0.1ml), the method is the same as step (2) in Example 1, and the yield is 87%. Melting point: 146°C.
[0145] By nuclear magnetic resonance spectrum analysis, the product obtained is 1,2-bis(methylthioethylphenyl)acetylene, and the specific data are: 1 HNMR (400MHz, CDCl 3 ): δ, 2.09(t, 6H, -CH 3 ), 2.73(t, 4H, -CH 2 -), 2.90(t, 4H, -CH 2 -), 7.08 (d, 4H, ArH), 7.41 (d, 4H, ArH).
[0146] (2) Hexa(4'-methylthioethylphenyl)benzene (HPB-(CH 2 ) 2 SC 1 )preparation
[0147] Using TOLAN-(CH 2 ) 2 SC 1 (1.31g, 4mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com