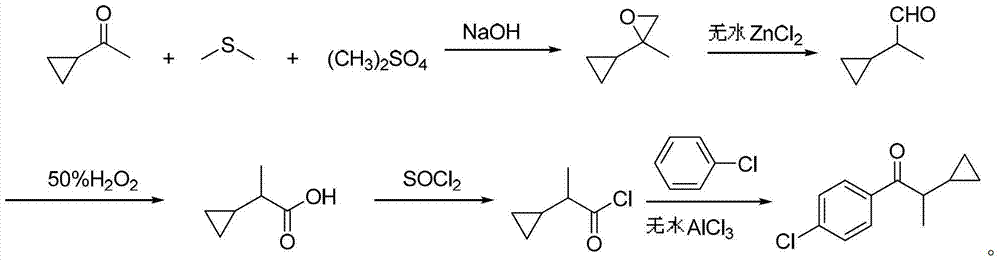
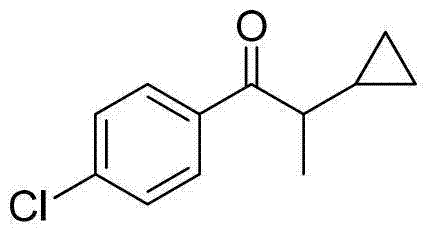
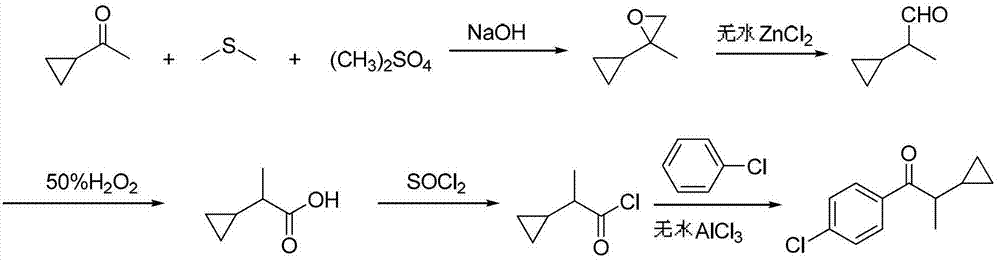
Preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
A technology of cyclopropyl propionic acid and cyclopropyl methyl ketone, which is applied in the field of preparation of 1--2-cyclopropyl-1-propanone, can solve the problem of strict water content in the reaction system, complicated operation, chlorophenylacetonitrile Easy self-polymerization and other problems, to achieve the effect of cheap and easy-to-obtain raw materials and solvents, high yield, and reduce three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 28.3g (0.22mol) of dimethyl sulfate, 136g of dimethyl sulfide, and 16.9g (0.20mol) of cyclopropylmethyl ketone into a 500mL three-necked flask with a stirring and reflux tube, reflux at 40°C for 1 hour, and dissolve the hydrogen Sodium oxide 17.8g (0.44mol) was added in portions within 1 hour, and the reflux reaction was continued for 2 hours. After the reaction is finished, neutralize with 10% dilute hydrochloric acid, stand to separate the phases, and reclaim dimethyl sulfide by distillation of the organic phase under normal pressure. 18.6 g of the still liquid is 2-cyclopropyl-2-methyloxirane, with a content of 96.2 % (gas chromatography), yield 93.2%.
[0030] Add 18.6g of 2-cyclopropyl-2-methyloxirane to a 100mL three-necked flask, add 0.6g of anhydrous zinc chloride at room temperature, stir for 4 hours, add 16g (0.23mol) of 50% hydrogen peroxide dropwise at room temperature , After adding in 1 hour, the reaction was continued for 3 hours, and the conversion ...
Embodiment 2
[0033] Add 28.3g (0.22mol) of dimethyl sulfate, 170g of dimethyl sulfide, and 16.9g (0.20mol) of cyclopropylmethyl ketone into a 500mL three-necked flask with a stirring and reflux tube, reflux at 40°C for 1 hour, and dissolve the hydrogen Sodium oxide 18.6g (0.46mol) was added in portions within 1 hour, and the reflux reaction was continued for 2 hours. Neutralize with 10% dilute hydrochloric acid after reaction finishes, stand to separate phases, organic phase normal pressure distillation reclaims dimethyl sulfide, still liquid 18.9g is 2-cyclopropyl-2-methyloxirane, content 96.5 % (gas chromatography), yield 95.0%.
[0034] Add 18.9g of 2-cyclopropyl-2-methyloxirane to a 100mL three-necked flask, add 0.7g of anhydrous zinc chloride at room temperature, stir for 6 hours, add dropwise 18.2g (0.26mol) of 50% Hydrogen peroxide was added in 1 hour, and the reaction was continued for 3 hours, and the conversion of 2-cyclopropylpropionaldehyde was complete as detected by liquid c...
Embodiment 3
[0037] Add 28.3g (0.22mol) of dimethyl sulfate, 170g of dimethyl sulfide, and 16.9g (0.20mol) of cyclopropylmethyl ketone into a 500mL three-necked flask with a stirring and reflux tube, reflux at 40°C for 1 hour, and dissolve the hydrogen Sodium oxide 19.4g (0.48mol) was added in portions within 1 hour, and the reflux reaction was continued for 3 hours. After the reaction is finished, neutralize with 10% dilute hydrochloric acid, stand to separate phases, and reclaim dimethyl sulfide by atmospheric distillation of the organic phase. 19.2 g of the still liquid is 2-cyclopropyl-2-methyloxirane, with a content of 96.1 % (gas chromatography), yield 94.1%.
[0038] Add 19.2g of 2-cyclopropyl-2-methyloxirane to a 100mL three-necked flask, add 0.96g of anhydrous zinc chloride at room temperature, stir for 6 hours, add dropwise 19.7g (0.28mol) of 50% Hydrogen peroxide was added in 1 hour, and the reaction was continued for 4 hours, and the conversion of 2-cyclopropylpropionaldehyde wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com