Polysaccharide and protein conjugate
A protein conjugation and conjugate technology, applied in the field of polysaccharide and protein conjugates, can solve the problems of weak immunogenicity, small molecular weight of LPS, and inability to produce immune boosting effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
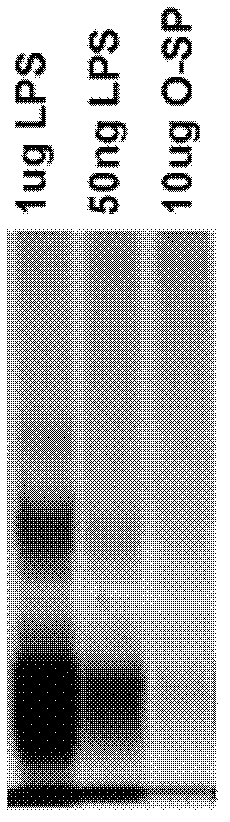
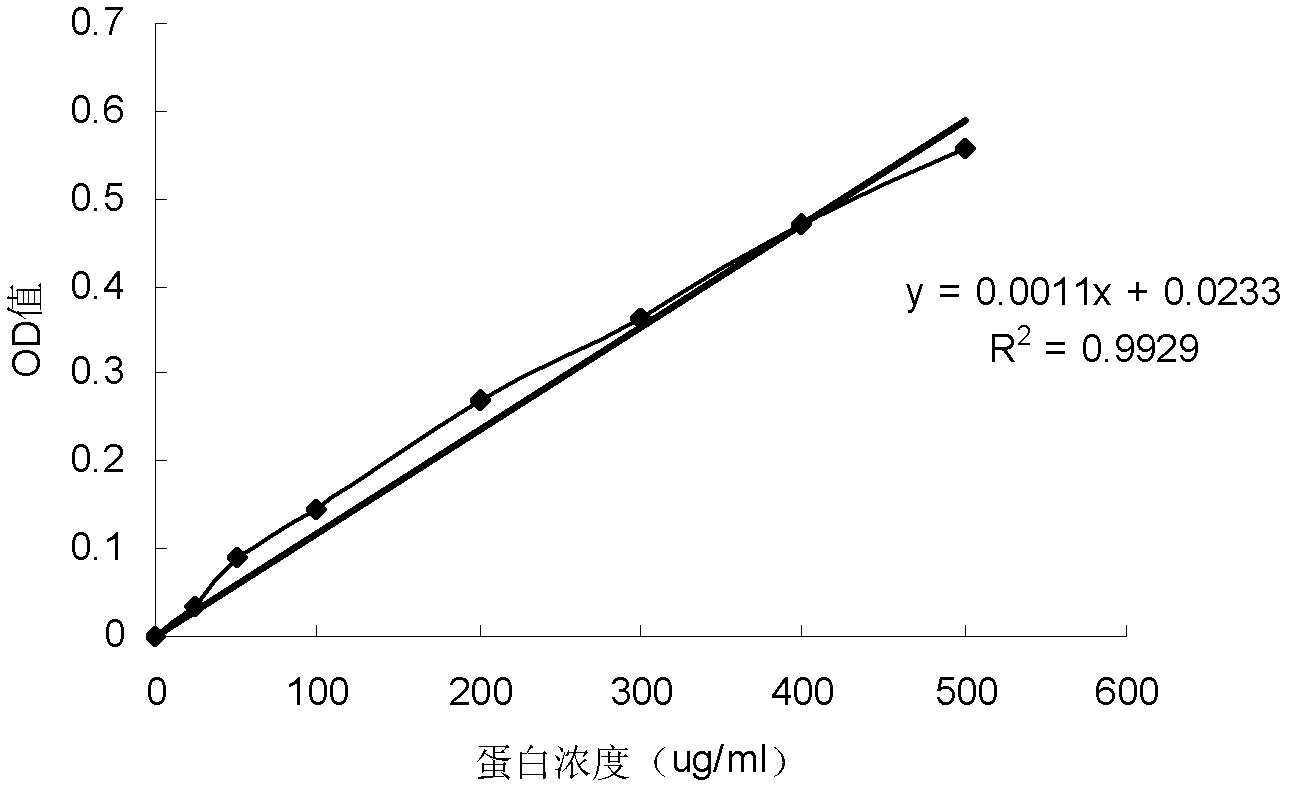
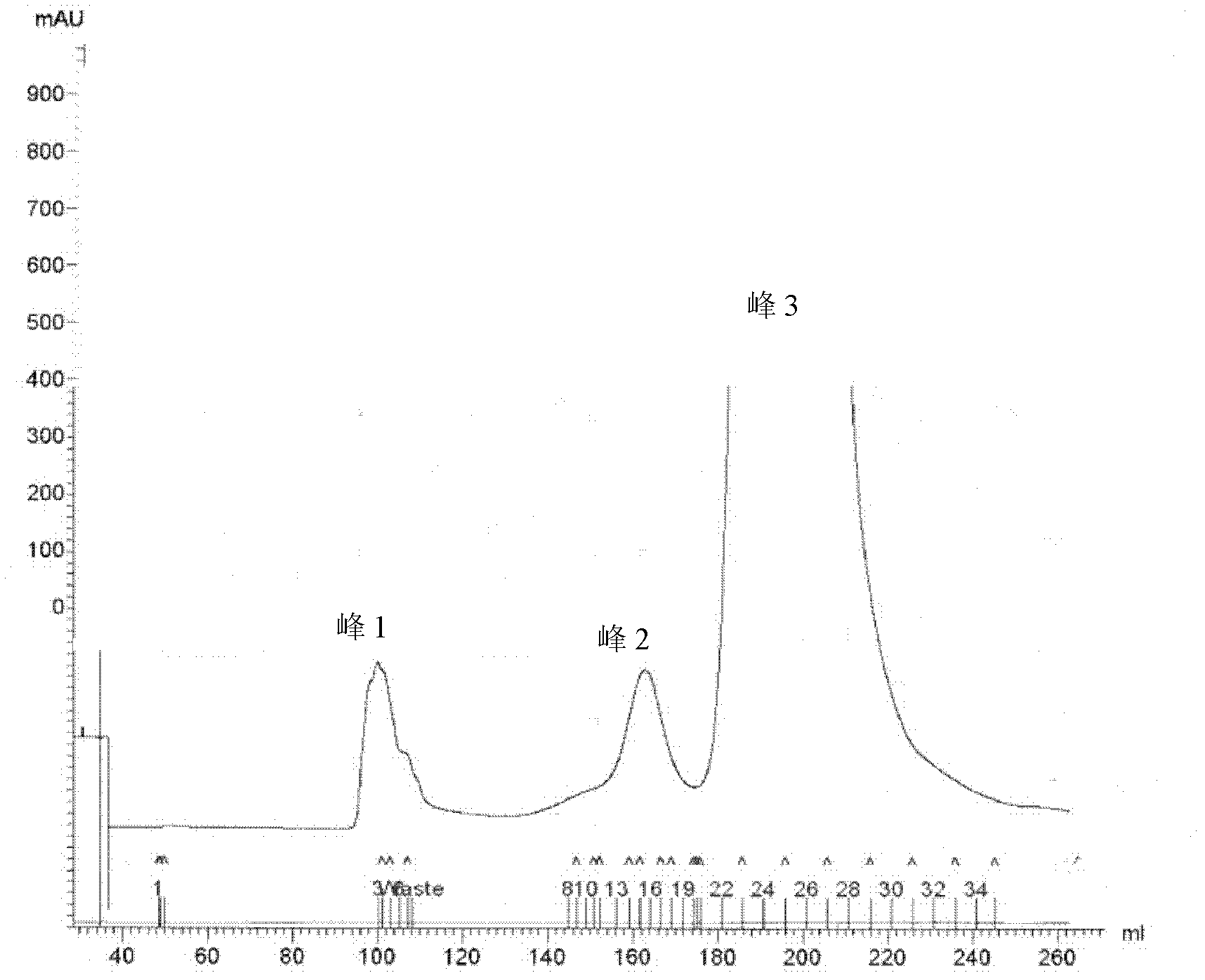
[0059] Embodiment 1, the preparation of the conjugate of polysaccharide and protein
[0060] I, extraction and purification of lipopolysaccharide (LPS)
[0061] 1. Extraction of LPS
[0062] The extraction method of LPS is recorded in Qin Min, Yang Guoyou, Zhou Fuchang, etc., Salmonella paratyphi A LPS extraction and O-SP purification. Progress in Microbiology and Immunology, 2005, 33 (01), 27-31, as follows:
[0063] Each time about 30 g of Vibrio cholerae 0139 (purchased from China National Institutes for Food and Drug Control) was added to 300 mL of 95% phenol aqueous solution, and then water was added until the total volume of the mixture was 600 mL. The mixed solution was shaken in a water bath at 68°C for 30 minutes, cooled in an ice bath, and then centrifuged at 7300×g and 10°C for 1 hour. Take the supernatant, add water to the remaining solution to a total volume of 600 mL, and repeat the 68°C water bath and centrifugation steps as described above. The operation was...
Embodiment 2
[0113] Embodiment 2, animal immunity experiment
[0114] 1. Immunity
[0115] Select 5-week-old BALB / c mice (BALB / C mice were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., female.) were randomly divided into two groups, namely the intraperitoneal injection group and nasal instillation group. Every big group is divided into 5 subgroups again, i.e. normal saline control group (normal saline concentration is 0.9% (mass percentage composition)), LPS (obtained by embodiment 1) immune group, CTB (obtained by embodiment 1) immune group , rBS-WC vaccine immunization group (rBS-WC vaccine was purchased from Shanghai United Cell Bioengineering Co., Ltd.) and LPS-CTB3 (obtained by Example 1, denoted as L-C) immunization group, with 6 mice in each group.
[0116] Mice in each group were immunized three times in total, and immunized on days 1, 15 and 29 (the first immunization was recorded as day 1). In the intraperitoneal injection group, the volume of e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Centrifugal force | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com