Medicine composition containing ergometrine and oxytocin analogue and preparation method
A technology of ergometrine and its composition, which is applied in the field of medicine, can solve problems such as unacceptable, inconvenient clinical use, and limited clinical application, and achieve the effect of reducing the production of related substances and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
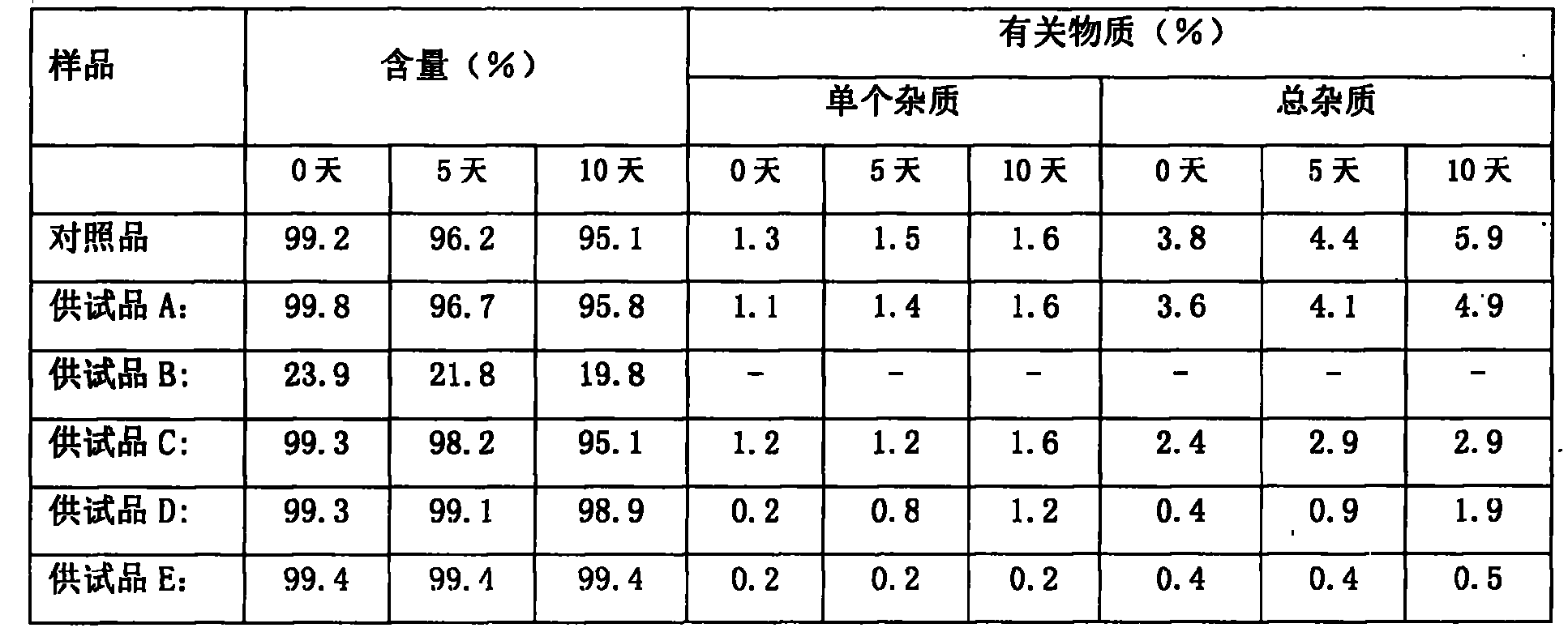
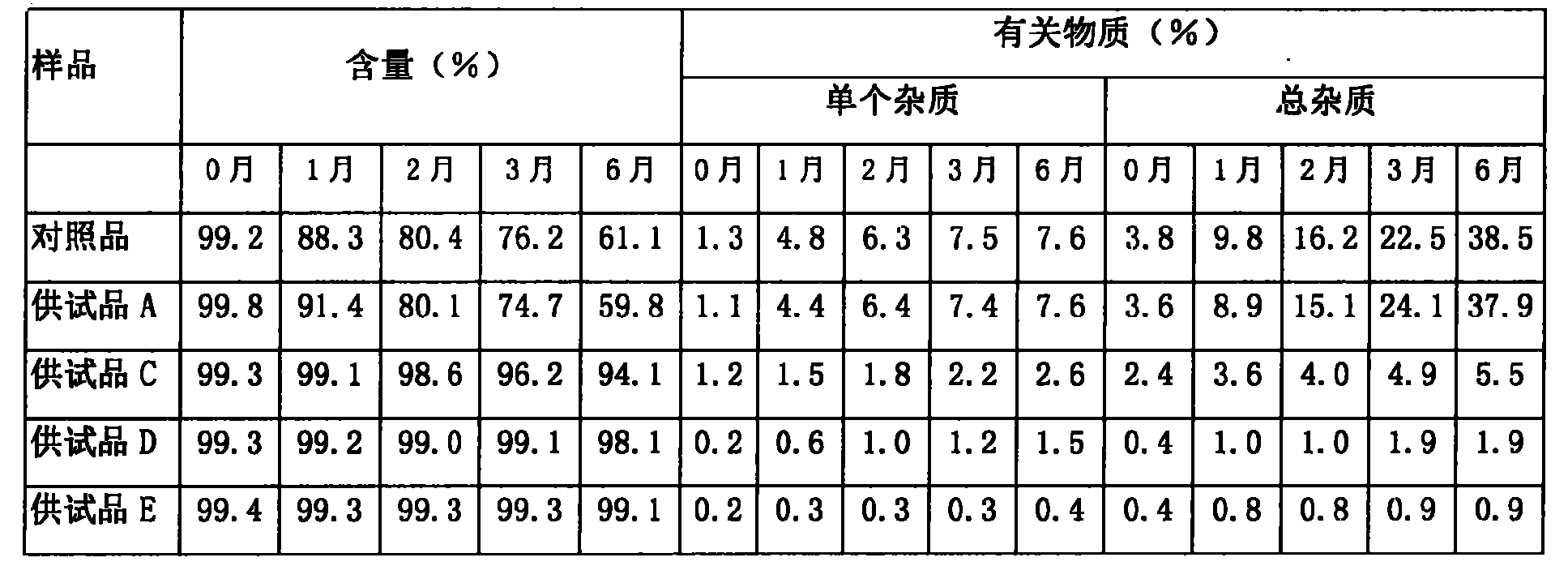
[0050] Comparison of content and stability of related substances in the pharmaceutical composition of compound oxytocin for injection and its analogs and pharmaceutically acceptable salt of ergometrine and ergometrine oxytocin injection
[0051] The test article and reference article:
[0052] Foreign reference substance: Syntometrine injection (ergometrine oxytocin injection), 10IU / ml / bottle, provided by Zuellig Pharma and produced by Novartis;
[0053] Test product A: Ergonovine Oxytocin Injection, imitating the prescription of foreign Syntometrine Injection, without antioxidant, produced by sterile filtration process, without high temperature sterilization, made by Nanjing Xianyu Technology Co., Ltd.;
[0054] Test product B: Ergonovine Oxytocin Injection, imitating the prescription of foreign Syntometrine Injection, does not contain antioxidants, and is sterilized at high temperature, provided by Nanjing Xianyu Technology Co., Ltd.;
[0055] Test product C: ergonovine oxy...
Embodiment 2
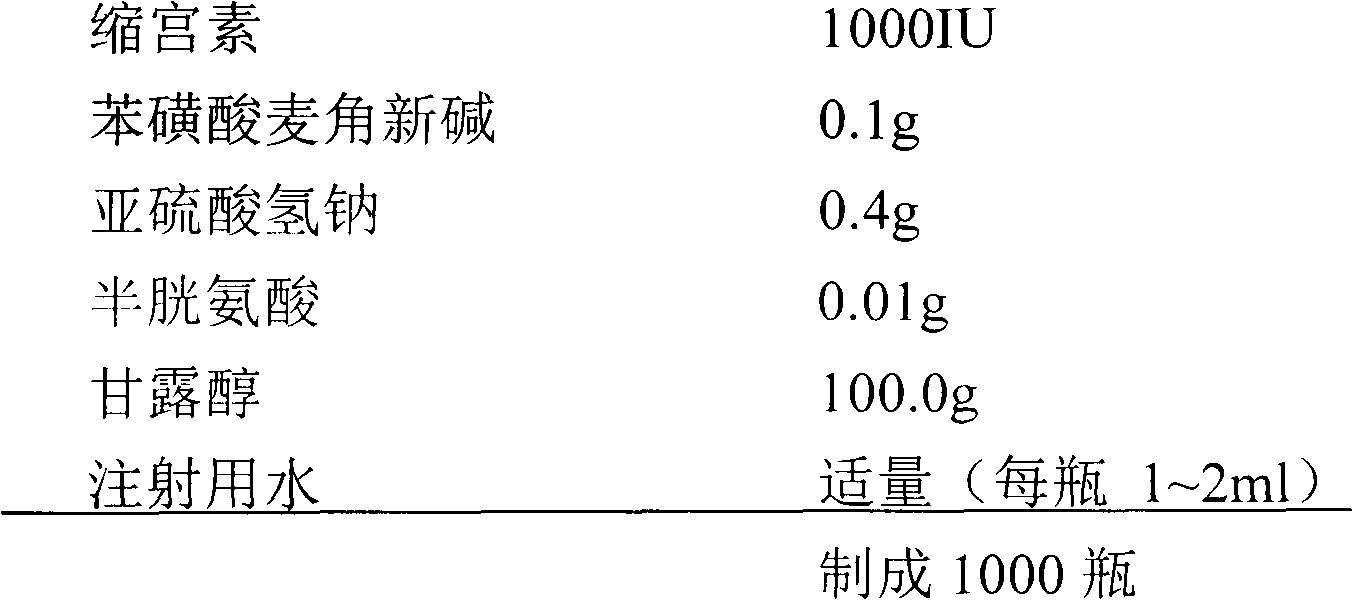
[0094] Compound ergometrine oxytocin for injection
[0095] (1) Prescription
[0096]
[0097] (2) Preparation process
[0098] Weigh the prescribed amount of mannitol, add 4 / 5 of the amount of water for injection, and stir to completely dissolve.
[0099] Use 1.0mol / L hydrochloric acid to adjust the pH value between 3-8, then add water for injection to make the volume to 1000ml.
[0100] Add 0.5 to 1 g of activated carbon, stir for 15 minutes, filter and remove carbon, add the prescribed amount of oxytocin, ergometrine besylate, sodium bisulfite and cysteine, and filter to sterilize with a 0.22um filter membrane.
[0101] Determination of the content of the main drug in the intermediate to determine the loading.
[0102] Freeze-dried, plugged and capped.
Embodiment 3
[0104] Compound Carbetocin Ergometrine Maleate for Injection
[0105] (1) Prescription
[0106]
[0107]
[0108] (2) Preparation process
[0109] Weigh the prescribed amount of dextran and glycine, add 90% of the total amount of water for injection, stir and dissolve with a 78HW-1 digital constant temperature magnetic stirrer to obtain solution (1).
[0110] Weigh 0.1% (W / V) activated carbon, add it to the above liquid (1) and stir for 15 minutes, decarbonize through an hourglass filter, and fine filter with a 0.22um filter membrane.
[0111] Take the combined antioxidant sodium metabisulfite and citric acid in the prescribed amount, slowly add them to 5% water for injection, stir to dissolve, and obtain the solution (2).
[0112] Take the prescribed amount of ergonovine and carbetocin and slowly add to (2), while stirring to completely dissolve.
[0113] Add the solution (2) to the solution (1), stir evenly, and adjust the pH to 4-6. Dilute to the mark with water f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com