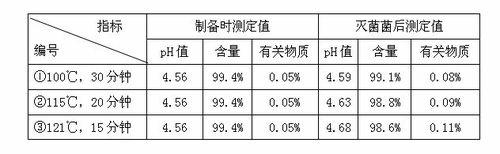
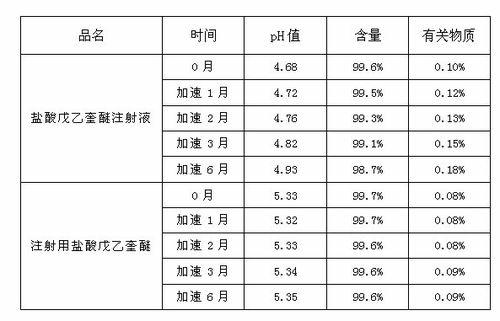
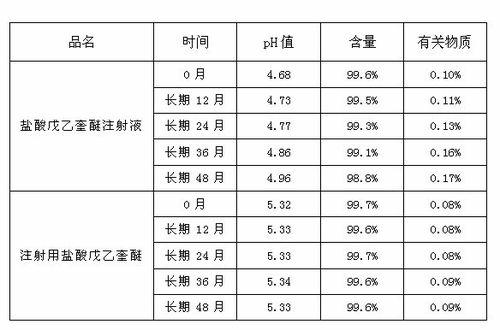
Preparation process of penehyclidine hydrochloride powder for injection
A penhyclidine hydrochloride, preparation technology, applied in the field of medical drugs, can solve the problems of content drop, pH value and related substances increase, and achieve the effects of reducing contact opportunities, related substances and content stability, and ensuring pH value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A, the preparation of penehyclidine hydrochloride aseptic solution
[0068] Weigh 0.1g of penehyclidine hydrochloride and 10g of mannitol, add 80ml of water for injection, add hydrochloric acid solution to adjust the pH value to 5.42, add water to 100ml, add 0.1g of activated carbon for needles, stir evenly, keep warm at 60°C for adsorption, first Decarburize by coarse filtration, and then filter with a 0.22 μm microporous membrane until clear to obtain the penhyclidine hydrochloride liquid.
[0069] B, freeze-drying of penehyclidine hydrochloride aseptic solution
[0070] a. Glass bottle treatment: Clean the glass bottle with ultrasonic waves, rinse it with pure water, then rinse it with water for injection, dry and sterilize it within 4 hours, and get a clean, sterile, dry, heat-free glass bottle; sterilization conditions And the method is: tunnel dry heat sterilization at 320°C for 5min.
[0071] b. Treatment of rubber stoppers: wash the rubber stoppers with dilute...
Embodiment 2
[0075] A, the preparation of penehyclidine hydrochloride aseptic solution
[0076] Weigh 0.1g of penehyclidine hydrochloride and 15g of mannitol, add 80ml of water for injection, add hydrochloric acid solution to adjust the pH value to 5.36, add water to 100ml, add 0.1g of activated carbon for needles, stir evenly, keep warm at 65°C for adsorption, first Decarburize by coarse filtration, and then filter with a 0.22 μm microporous membrane until clear to obtain the penhyclidine hydrochloride liquid.
[0077] B, freeze-drying of penehyclidine hydrochloride aseptic solution
[0078] a. Glass bottle treatment: Clean the glass bottle with ultrasonic waves, rinse it with pure water, then rinse it with water for injection, dry and sterilize it within 4 hours, and get a clean, sterile, dry, heat-free glass bottle; sterilization conditions And the method is: dry heat sterilization at 180°C for 1.5h.
[0079] b. Treatment of rubber stoppers: wash the rubber stoppers with dilute hydroc...
Embodiment 3
[0083] A, the preparation of penehyclidine hydrochloride aseptic solution
[0084] Weigh 0.1g of penehyclidine hydrochloride and 20g of xylitol, add 80ml of water for injection, add hydrochloric acid solution to adjust the pH value to 8.0, add water to 100ml, add 0.1g of activated carbon for needles, stir evenly, and keep it at 70°C for adsorption. Decarbonize by coarse filtration first, and then filter with a 0.22 μm microporous membrane until it becomes clear to obtain the penhyclidine hydrochloride medicinal solution.
[0085] B, freeze-drying of penehyclidine hydrochloride aseptic solution
[0086] a. Glass bottle treatment: Clean the glass bottle with ultrasonic waves, rinse it with pure water, then rinse it with water for injection, dry and sterilize it within 4 hours, and get a clean, sterile, dry, heat-free glass bottle; sterilization conditions And the method is: tunnel dry heat sterilization at 320°C for 5min.
[0087] b. Treatment of rubber stoppers: wash the rubb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com