Thymalfasin liposome preparation for injecting
A new technology of liposome preparation and thymus method, which is applied in the field of medicine, can solve the problems of unspecified mannitol stabilizer scheme and test data, cumbersome preparation process of thymosin injection, and unsuitability for industrial production, etc., so as to improve bioavailability Degree, improve the quality of preparation products, increase the effect of retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
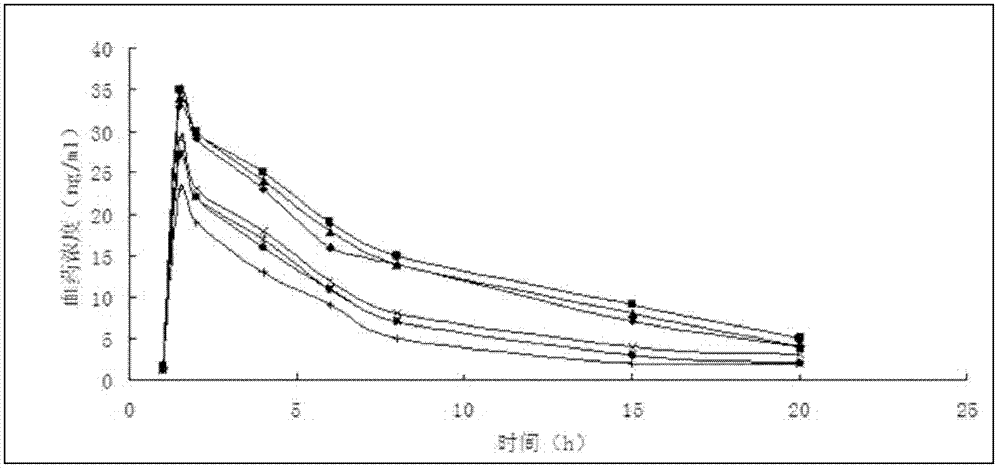
Image
Examples
preparation example Construction
[0068] On the other hand, the present invention also provides a preparation method of thymus method new liposome preparation for injection, specifically comprising the following preparation steps:
[0069] (1) Put cholesterol, egg yolk lecithin, phosphatidylinositol and sucrose ester in a pear-shaped bottle, add an organic solvent, heat and stir to dissolve, then remove the organic solvent under reduced pressure on a rotary evaporator, and form on the bottle wall Uniform and transparent phospholipid film;
[0070] (2) Under the protection of nitrogen, add thymofaxin aqueous solution into the bottle and stir to elute the phospholipid membrane and fully swell and hydrate it. After the hydration is complete, do gradient homogenization at 100bar to 600bar for 4 to 6 times, 0.22μm The new liposome of the thymus method is obtained by filtering through a pore filter membrane;
[0071] (3) Under sterile conditions, dissolve the prescribed amount of trehalose and mannitol in water for...
Embodiment 1
[0078] The preparation of embodiment 1 injection thymus method new liposome preparation
[0079] The components and weights of the raw and auxiliary materials used are as follows:
[0080]
[0081]
[0082] The new liposome preparation of thymus method for injection is prepared by preparation process:
[0083] (1) 60g cholesterol, 180g egg yolk lecithin, 30g phosphatidylinositol and 20g sucrose ester S15 are placed in a pear-shaped bottle, added to 10000ml chloroform, heated and stirred to make it dissolve, and then the chloroform is removed under reduced pressure on a rotary evaporator, Form a uniform and transparent phospholipid film on the bottle wall;
[0084] (2) Under the protection of nitrogen, add 1.6g of thymus fasin dissolved in 500ml of water for injection into the bottle, stir to elute the phospholipid membrane and fully swell and hydrate it. Massage 4 to 6 times, and filter through a 0.22 μm microporous membrane to obtain thymofaxin liposomes;
[0085] ...
Embodiment 2
[0086] The preparation of embodiment 2 thymus method new liposome preparations for injection
[0087] The components and weights of the raw and auxiliary materials used are as follows:
[0088]
[0089] The new liposome preparation of thymus method for injection is prepared by preparation process:
[0090] (1) 70g cholesterol, 220g egg yolk lecithin, 40g phosphatidylinositol and 30g sucrose ester S15 are placed in a pear-shaped bottle, added to 10000ml chloroform, heated and stirred to make it dissolve, and then the chloroform is removed under reduced pressure on a rotary evaporator, Form a uniform and transparent phospholipid film on the bottle wall;
[0091] (2) Under the protection of nitrogen, add 1.6g of thymus fasin dissolved in 500ml of water for injection into the bottle, stir to elute the phospholipid membrane and fully swell and hydrate it. Massage 4 to 6 times, and filter through a 0.22 μm microporous membrane to obtain thymofaxin liposomes;
[0092] (3) Und...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com