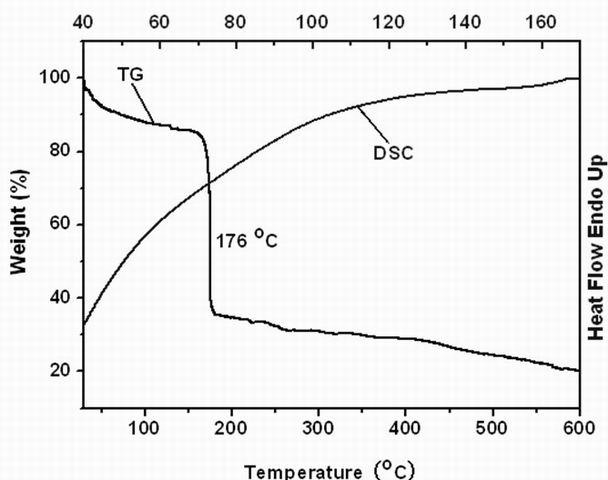
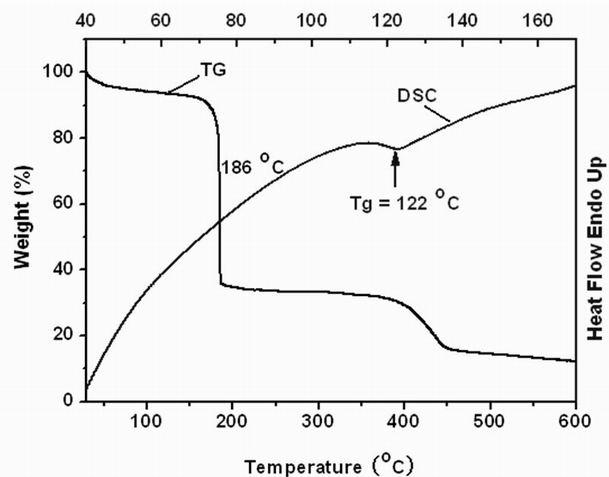
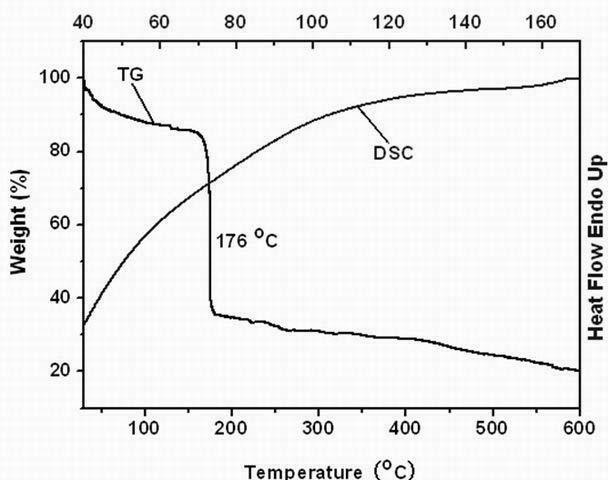
Biphenyl molecular glass and preparation method thereof
A technology of molecular glass and biphenyl type, which is applied in the field of polyphenol biphenyl type compound and its synthesis, can solve the problems of chain entanglement and high line width roughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The synthetic route of compound A and compound B is as follows:
[0028]
[0029] Using m-bromonitrobenzene and 3,5-dimethoxybromobenzene as raw materials, Compound A was synthesized through 6 steps, and Compound B was synthesized through 7 steps. 2,2'-dibromo-4,4'-biphenyldiamine was rearranged under the conditions of m-bromonitrophenyl zinc powder, and the compound 3,5-dimethoxybromobenzene was reacted with magnesium chips to obtain Grignard After reagents, add to B(OMe) at low temperature 3 3,5-dimethoxyphenylboronic acid, 2,2'-dibromo-4,4'-biphenyldiamine and 3,5-dimethoxyphenylboronic acid were obtained in Pd(PPh 3 ) 4 / K 2 CO 3 Catalytic Suzuki reaction gives 2,2'-bis(3,5-dimethoxyphenyl)-4,4'-biphenylenediamine. 2,2′-bis(3,5-dimethoxyphenyl)-4,4′-biphenyldiamine in NaNO 2 / H 2 SO 4 The diazotization reaction occurs under certain conditions, and then heated and hydrolyzed under acidic conditions to obtain 2,2'-bis(3,5-dimethoxyphenyl)-4,4'-biphenol. Ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com