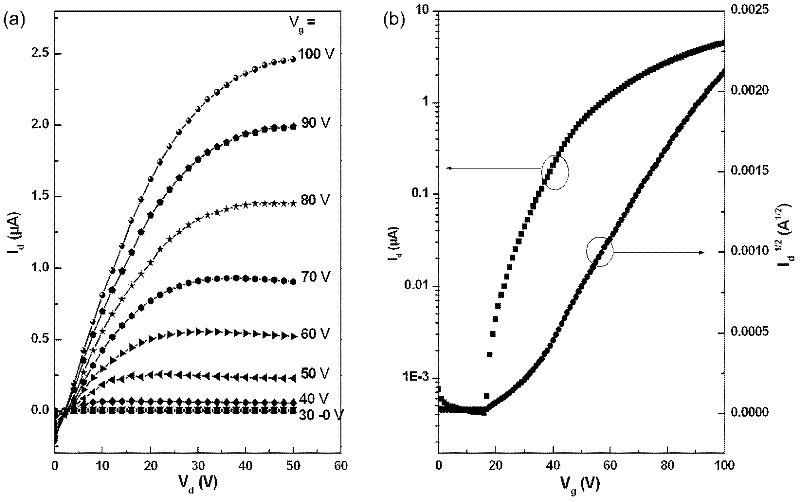
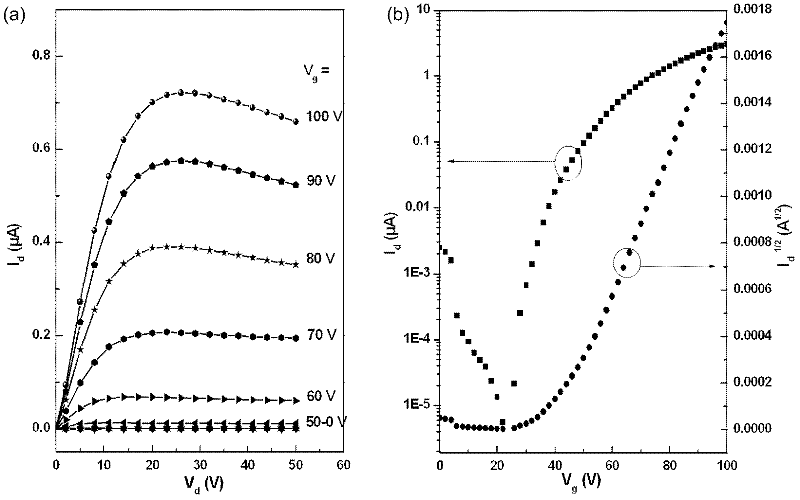
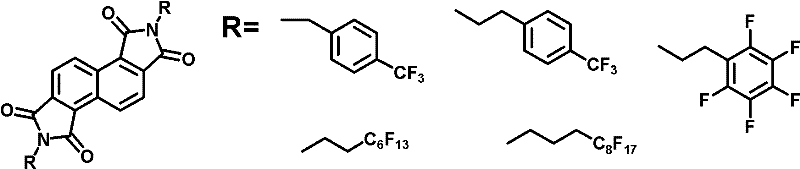
N-type organic semiconductor material containing oblique line type naphthalimide unit
An organic semiconductor and diimide technology, which is applied in the field of n-type semiconductor materials, can solve the problems of lack of high electron mobility, air-stable n-type organic semiconductor materials, and few types.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0020] Preparation 1: 1,2,5,6-naphthalene tetracarboxylic acid
[0021] Add 0.85 g of 1,5-dicyano-2,6-dimethylnaphthalene and 20 mL of K 2 Cr 2 o 7 (3.56g) and NaOH (0.33g) in water. After the mixture was stirred for half an hour, it was sealed in a stainless steel jar, heated to 220°C and maintained for 10 hours. After cooling, it was filtered, and the filtrate was treated with ether, and the aqueous layer was acidified by adding concentrated hydrochloric acid to obtain a yellow solid (1.06 g, yield 85%). Preparation 2: 1,2,5,6-naphthalene tetracarboxylic anhydride
preparation example 2
[0022] Add 1.0 g of 1,2,5,6-naphthalene tetracarboxylic acid obtained in the previous step into 50 mL of acetic anhydride, stir and reflux in a 100 mL round bottom flask for 3 hours, distill off most of the solvent under reduced pressure, vacuum filter, and the residue After washing with methanol, 0.79 g of product was obtained with a yield of 90%.
preparation example 3
[0023] Preparation Example 3: N, N'-bis(4-(trifluoromethyl)phenyl)-naphthalene-1,2,5,6-bis(dicarboxamide)
[0024]
[0025] The acid anhydride (0.586g, 2.2mmol) obtained in the previous step reaction and excess 4-trifluoromethylbenzylamine (6.6mmol) and zinc acetate (1.54mmol) were added in a 25mL round bottom flask, and 6mL quinoline was added, Heating to above 200°C, stirring and reacting at this temperature for 3 hours. After cooling, vacuum filter, and successively use hot dilute Na 2 CO 3 solution, water, toluene, methanol wash. 1.06 g of crude product were obtained, yield 83%. The further extraction of the product was purified by vacuum sublimation. Elemental analysis: calcd for C, 61.86; H, 2.77; N, 4.81; found: C, 62.03; H, 2.77, N, 4.87.Mp 317-318℃.MALDI-TOF / MS, m / z calcd for C 30 h 16 f 6 N 2 o 4 :582.1, found: 582.1; 1 H NMR (400MHz, CDCl 3 , ppm): δ=4.97(s, 4H), 7.60(s, 8H), 8.12(d, J=8.4Hz, 2H), 9.35(d, J=8.4Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com